Our lab has interdisciplinary biomedical engineering research programs focusing on the development of novel optical imaging methods for biomedical applications, including functional neuroimaging, early cancer detection, transplant organ evaluation, and tissue engineering. Our optical imaging technologies include optical coherence tomography (OCT), multi-photon microscopy (MPM), fluorescence molecular imaging (FMI), laminar optical tomography (LOT), and endoscopy.
Technology Development
Optical Coherence Tomography (OCT)
Optical coherence tomography (OCT) is an emerging medical diagnostic imaging technology that enables micron-scale, cross-sectional imaging of microstructure in biological tissues in situ and in real time. OCT imaging is analogous to B-mode ultrasound, except that OCT is performed by measuring the echo time delay and intensity of reflected or backscattered light rather than sound. An optical beam is scanned across the tissue and the backscattered light is measured as a function of axial depth and transverse position. In this way, OCT can generate cross-sectional, tomographic images of subsurface tissue microstructure. Three-dimensional tissue morphology can be formed by stacking a series of two-dimensional OCT tomograms. OCT can perform imaging with resolutions approaching that of conventional histopathology, but without the need of tissue removal. Furthermore, we developed the GRIN-rod-lens based needle-imaging probe (O.D. = 740 μm). High-speed (100 frames/s) and high-sensitivity (>90 dB) OCT imaging was achieved by using this needle probe. The stationary GRIN-rod-lens also provides high quality DOCT imaging with 41 dB velocity dynamic range (VDR) and ± 17 um/s velocity resolution . Polarization-sensitive optical coherence tomography (PS-OCT) is a functional extension of optical coherence tomography (OCT), which is particularly useful when the nano-scale organization of tissue are difficult to be observed in the intensity images of a regular OCT. Our lab developed a simple but effective method to quantitatively measure the birefringence of tissue by an all single-mode fiber (SMF) based polarization-sensitive optical coherence tomography (PS-OCT) with single input polarization state.
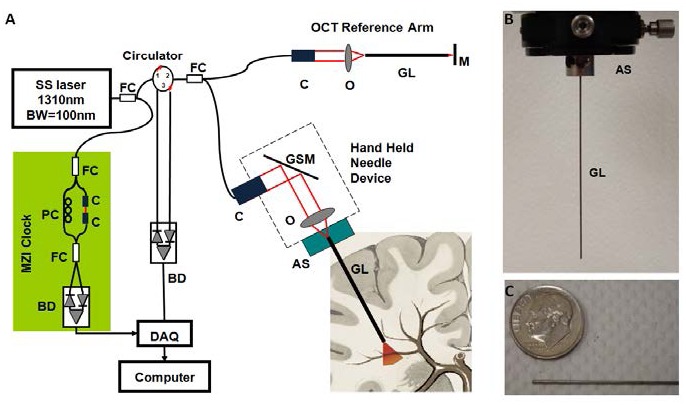
(A) Schematic of the handheld OCT system. FC: fiber coupler; PC: polarization controller; C: collimator, BD: balanced detector, MZI: Mach-Zehnder interferometer (frequency clocks), DAQ: data acquisition board, M: mirror, GSM: galvonometer scanning mirror, O: objective lens, AS: alignment stages, GL: GRIN lens needle. (B) GRIN needle. (C) GRIN needle placed beside a U.S. dime.
Fluorescence Laminar Optical Tomography (FLOT)
Laminar optical tomography (LOT) is a promising optical imaging method, which detects the scattered light from different depths simultaneously by using an array of detectors with different separations from the light source. LOT has a resolution of ~100 um with 2-3 mm penetration depths. LOT was initially utilized to image hemodynamic changes based on absorption contrast. Soon after , LOT was rapidly adapted for fluorescent molecular imaging, which was termed either fluorescence laminar optical tomography (FLOT), or mesoscopic fluorescence molecular tomography (MFMT), and several improvements or alternative system designs have been investigated . We also built angled FLOT system since angled illumination and detection configurations has been shown improving both resolution and depth sensitivity.
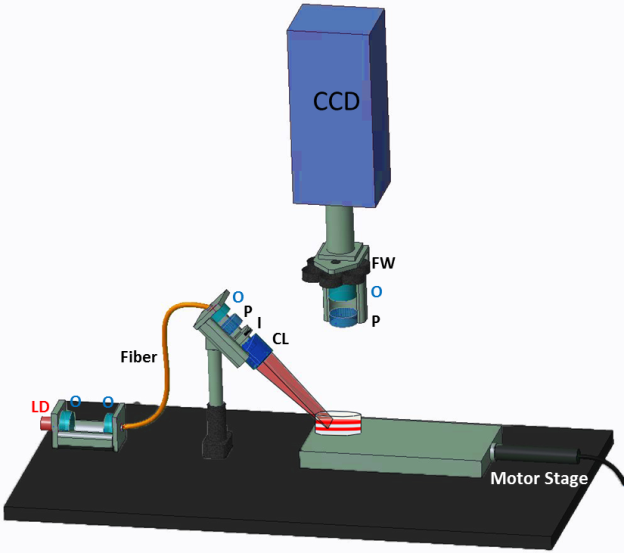
Schematic of the FLOT system. O: objective lens, LD: laser diode, P: polarizer, S: shutter, I: iris, CL: cylindrical lens, FW: filter wheel.
Multi-photon Microscopy (MPM)
Two-photon microscope (TPM), based on the simultaneous absorption by each fluorophore molecule of two photons in a single quantum event, offers many advantages over conventional imaging techniques and has become a powerful tool for studying biological function in living tissues.
Biomimetic Phantom Technology
We collaborate with Dr. Joshua Pfefer at FDA to investigate novel phantom-based test methods for assessment of optical imaging/spectroscopy systems, including OCT, hyperspectral imaging, FMI, and near-infrared spectroscopy (NIRS). Currently, we are investigating novel 3D-printed bio-mimetic phantom for characterization and validation of FMI imaging systems.
Biomedical Applications
Neuroimaging
Depth-resolved Imaging of Neural Connections in Sensory and Motor Cortices
The primary somatosensory (S1) and primary motor (M1) cortices are reciprocally connected, and their interaction has long been hypothesized to contribute to coordinated motor output. However, very little is known about the nature and synaptic properties of the S1 input to M1 . Neuroanatomical tracing experiments, optogenetic stimulation methods, and electrophysiological studies have shown that neurons in S1 target neurons in L2/3 and L5 of M1, while with a distinct band between L5 and L2/3 containing neurons that were not labeled as shown in Figure 1(A-B) from ex-vivo study . Most of the current optical imaging setups involving CCD cameras can only provide two-dimensional information and cannot detect depth-resolved neuronal activation in vivo.
FLOT is a promising technology to investigate depth-resolved sensorimotor interaction in vivo. Our group has demonstrated the feasibility of FLOT for depth-resolved brain imaging at ~100 ïm resolution with 1-2 mm imaging depth. Time-resolved FLOT combined with voltage-sensitive dye imaging(VSDi) enables fast three-dimensional mesoscopic imaging of neural activities evoked in the barrel and motor cortex, following deflection of a single whisker with 5 ms temporal resolution. We will refine the technology by developing high-dynamic-range FLOT using multi-exposure schemes to extend the image depth, and apply to mouse model to study the dynamic neural activities in 3D. In addition, we will adapt optogenetic methods to validate the layer-specific connections between S1 and M1 cortices. Furthermore, LOT is also possible to provide hemodynamic responses induced by neuronal activation . Therefore it has strong potential to investigate the neuronal and vascular responses that underlie the functional changes to further elucidate neurovascular coupling. Furthermore, such functional map can be overlaid with OCT angiography for anatomical co-registration and Doppler OCT for blood flow information.
This work is in collaboration with Prof. Reha Erzurumlu at UMB.
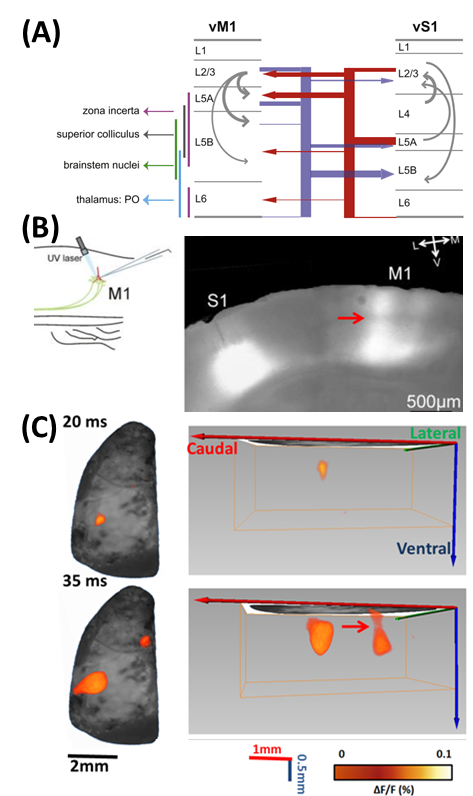
(A) Long-range circuits connecting vS1 and vM1. From [1]. (B) Optogenetic stimulation of the S1-to-M1 pathway. Adeno-associated virus (AAV) was injected in the S1 of animals in vivo. Infected S1 cells expressed channel-rhodopsin (ChR). M1 cells were recorded using a laser beam to activate ChR-expressing terminals originating in S1. ChR expression can be seen along axons traveling to M1, and within M1 itself. Red arrow indicates a distinct band between L5 and L2/3. From [2]. (C) Left column shows 2D imaging of changes in fluorescence in response to C2 whisker stimulation. Right column shows corresponding 3D fluorescence changes in mouse brain using FLOT. Post-stimulation time is indicated at the left side of each image. Red arrow indicates the distinct band between L5 and L2/3 corresponding to the band shown in (B).
Multi-scale Functional Brain Imaging
Our group has developed a forward-viewing needle imaging device (OD < 1 mm) for both OCT and fluorescence imaging. This technology enables high-resolution imaging of neural structure and function in deep brain. In collaboration with Prof. Reha Erzurumlu at UMB, we have demonstrated needle-based VSDi in thalamus in response to whisker stimulation. Currently we are developing multi-channel optical imaging systems for simultaneous imaging of cortical and thalamus response to investigate corticothalamic pathways for sensory processing.
[caption id="attachment_43" align="aligncenter" width="652"]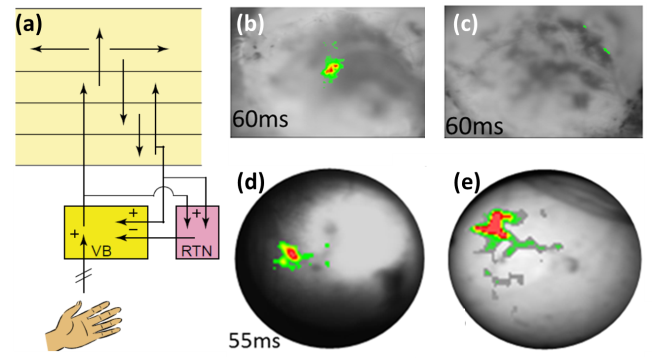 (A) Schematics of the corticothalamic pathways[3]. (B) Voltage-sensitive dye optical images showing C2 whisker stimulation evoked changes in cortex before and (C) after muscimol injection (blocking corticothalamic feedback). Color bar: ΔF/F = 0-0.1%. (D) Needle-based VSDi showing C2 whisker stimulation evoked changes in thalamus before and (E) after muscimol injection. Color bar: ΔF/F = 0-0.1% in (D) and 0-0.06% in (E). [/caption]
(A) Schematics of the corticothalamic pathways[3]. (B) Voltage-sensitive dye optical images showing C2 whisker stimulation evoked changes in cortex before and (C) after muscimol injection (blocking corticothalamic feedback). Color bar: ΔF/F = 0-0.1%. (D) Needle-based VSDi showing C2 whisker stimulation evoked changes in thalamus before and (E) after muscimol injection. Color bar: ΔF/F = 0-0.1% in (D) and 0-0.06% in (E). [/caption]
To overcome the limited field-of-view of needle-based imaging, we integrated high-resolution optical imaging probe with large field-of-view MRI for multi-scale imaging and image-guided interventions. We will interface optical imaging with functional MRI and diffusion tenser MRI (DTI) for multi-scale imaging of brain structure and function. We will include multiple optical imaging modalities (Doppler OCT for blood flow, polarization-sensitive OCT for fiber orientation, and hyperspectral imaging for oxygen saturation) to obtain multi-parametric information during brain activation. Successful development of such multi-scale MRI-optical imaging system may yield a minimally-invasive diagnostic tool for neural diseases in the future.
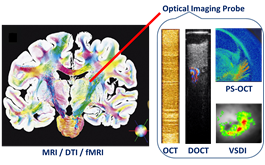
Schematics of the proposed multi-scale, multi-modal neuroimaging platform.MRI image in the left is from Ref. [4].
Imaging Renal Physiology and Transplant Kidney Viability
Single Nephron Imaging
In collaboration with Dr. Peter Andrews from Georgetown University Medical School, we demonstrated the feasibility of OCT and Doppler OCT in imaging the microanatomy and microcirculation of kidney .
Our group has developed multi-modal microscopy (optical coherence microscopy, OCM, and multi-photon microscopy, MPM) for kidney imaging in vivo. Such imaging system will provide quantitative multi-parametric imaging information for kidney pathologies, including glomerular and tubular from OCM and blood flow and glomerular filtration from MPM. This technology can be also applied to study vascular/glomerular/tubular alterations associated with other kidney diseases, such as diabetic nephropathy.

(A-C) OCT and Doppler OCT (DOCT) imaging of a rat glomerulus in vivo at depths of 470 um. A: OCT en face views of single glomerulus. B: DOCT en face view of the same imaging plane. C: fused OCT/DOCT image
Clinical Imaging of Transplant Kidney Viability
In addition, we translated this technology to clinical practice by imaging transplant kidney viability using OCT in an ongoing clinical study performed at the Georgetown University Medical Center. Based on the research results, we suggest a modified clinical protocol to better protect transplant kidneys. Currently our research is focused on advanced image processing methods to quantitatively assess the morphometric parameters (e.g., tubular lumen diameters) and functional parameters (microvascular blood flow) as indicators of the functional status of transplanted kidneys.
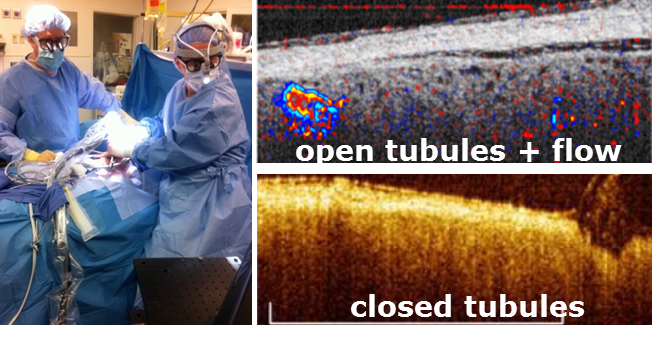
(Left) Transplant surgeons holding our OCT probe during kidney transplant surgery. (Right) OCT/DOCT of human kidney.
Multimodal Cancer Imaging and Translational Devices
OCT/FLOT Imaging of Colon Cancer
One unique aspect of our research is the combination of OCT and fluorescence molecular imaging (FMI), as OCT provides high-resolution morphological information and fluorescence imaging offers high-sensitivity molecular information. Together, such unique combination enables more comprehensive characterization of biological tissues. In collaboration with Dr. Ronald Summers at NIH Clinical Center, our group demonstrated co-registered OCT and fluorescence molecular imaging of mouse colon adenoma. Furthermore, we integrated OCT with depth-resolved fluorescence laminar optical tomography (FLOT), which enables mesoscopic (mm-scale) morphological and molecular tomography. The hybrid OCT/FLOT imaging holds great promise as a powerful tool for diagnosis of early cancers.
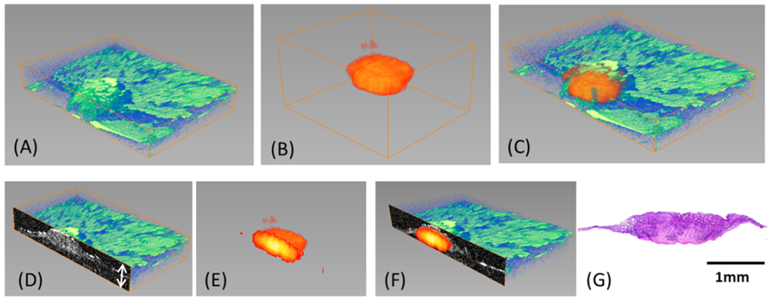
(A)3D OCT image (XxYxZ = 5x3x0.5 mm²) (B)3D FLOT images(XxYxZ =3.8×3.5×2 mm²) (C)3D OCT and FLOT fused image(D)Cross-sectional OCT images(E)Cross-sectional FLOT images(F)2D OCT and FLOT fused image(G)Corresponding Histology
Real-Time Monitoring of Photoimmunotherapy
In addition, our group also investigated tumor microenvironment changes during cancer therapy (photoimmunotherapy, PIT) in collaboration with Dr. Hisataka Kobayashi at NCI. In our pilot studies, optical coherence tomography (OCT) reveals dramatic hemodynamic changes during PIT. We developed and applied speckle variance analysis, Doppler flow measurement, bulk motion removal, and automatic region of interest selection to quantify vessel diameter and blood velocity within tumors in vivo. We are appling a minimally-invasive two-channel fluorescence fiber imaging system by combining the traditional fluorescence imaging microscope with two imaging fiber bundles to monitor mAb-IR700 distribution and therapeutic effects during PIT at different intra-tumor locations (e.g. tumor surface vs. deep tumor) in situ and in real time simutaneously, thereby enabling evaluation of the therapeutic effects in vivo and optimization of treatment regimens accordingly.

Vascular changes in a photoimmunotherapy (PIT)-treated tumor blood vessel. (a to c) Representative OCT/DOCT/SV images at different time points (indicated by the orange dashed lines) during the PIT treatment. (d) Time plot of the blood velocity (red line) and the vessel width (blue line). In the first 60 s (indicated by the black dashed line), the laser light is off to obtain baseline information. After 60 s, near-infrared (NIR) laser light is turned on for 10 min (60 to 660 s). The dosage is 100J∕cm2. In the last 60 s (660 to 720 s), the light is turned off to obtain post-treatment information (indicated by the second black dashed line on the right).
Image-guided Biopsy Needle for Cancer Diagnosis and Prognosis
We collaborate with University of Pennsylvania School of Medicine to develop novel image-guided biopsy needle for breast cancer diagnosis and prognosis. The central hypothesis is that heterogeneity-associated mitochondrial redox imaging indices (FAD, NADH and redox ratio) can predict the metastatic potential of breast cancer. Our group has developed an imaging-fiber-bundle based intrinsic fluorescence imaging probe (2 mm in diameter), and will integrate into standard 12-Gauge vacuum-pump biopsy needle. This device will be subsequently tested on animal models and human patients.
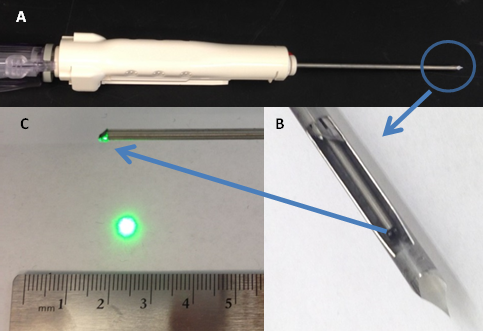
Photographs of (A) 12-Gauge vacuum-pump biopsy needle, (B) Side-viewing GRIN needle probe inside the biopsy needle. 1-mm diameter GRIN rod lens needle probe with a prism at the tip of the GRIN rod lens needle probe to turn the green illumination light to 90 degree as shown in (C).
Image-Guided Optimization of Tissue Engineering Scaffolds
We have combined OCT and fluorescence confocal microscopy to characterize scaffold designs for engineered tissues and monitor stem cell behaviors. In this project, we will further develop a 3D multi-modality tomographic imaging platform that combines depth-resolved biochemical/molecular information from FLOT with the morphological/architectural information from OCT to monitor cell viability, migration, proliferation, differentiation, and signaling within regenerative medicine constructs in situ. This project will result in a new non-destructive imaging technology for quantitative characterization of cell-scaffold interactions, which is essential to understand the fundamental mechanisms in tissue engineering and regenerative medicine, and will also enable optimized design and materials of tissue engineering scaffolds, cell-seeding methods, and chemical/environmental cues.
We will image stem-cell-laden tissue scaffolds to examine the effects of scaffold architecture on hMSC differentiation (specifically, the role of porosity, pore size, and interconnectivity on the expression of osteogenic growth factors that will lead to rapid and efficient osteoblastic differentiation). In addition, we will image the dynamics of hMSC behavior within scaffolds to describe which architectures support rapid cell infiltration and differentiation. Next, the effects of different scaffolds on in vivo bone formation will be observed in a pre-clinical animal model of cranial defect. We will use in vivo outcomes on animal model as a feedback to derive the optimal optical imaging criteria describing scaffold characteristics. This work is in collaboration with Prof. John Fisher at UMD.

3D LOT tomograms of fluorescent-labeled hMSCs (green-blue color scale) in alginate scaffolds (grey color scale) at day 0 and 21, respectively. FOV A: 4.6 x 1.6 x 2.6 mm3, B: 4.6 x 2.3 x 3.7 mm3.
References
1. T. Mao, D. Kusefoglu, B.M. Hooks, D. Huber, L. Petreanu, and K. Svoboda, Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron, 72(1): p. 111-23 (2011).
2. I. Petrof, A.N. Viaene, and S.M. Sherman, Properties of the primary somatosensory cortex projection to the primary motor cortex in the mouse. J Neurophysiol, 113(7): p. 2400-7 (2015).
3. H.J. Alitto and W.M. Usrey, Corticothalamic feedback and sensory processing. Curr Opin Neurobiol, 13(4): p. 440-5 (2003).
4. M. Axer et al., Neuroimage 54, 1091-1101 (2011).
