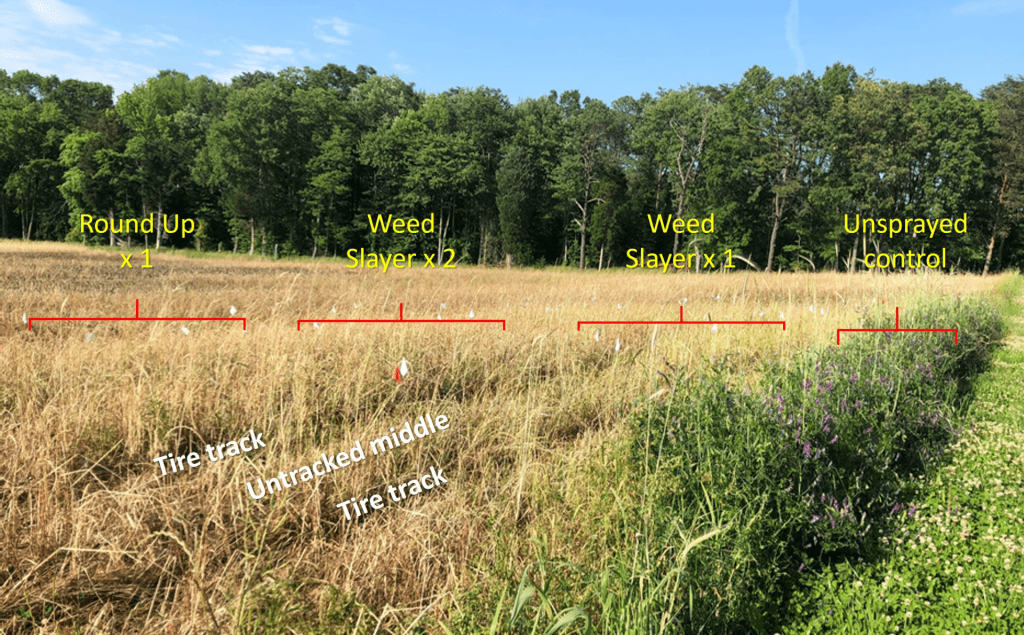
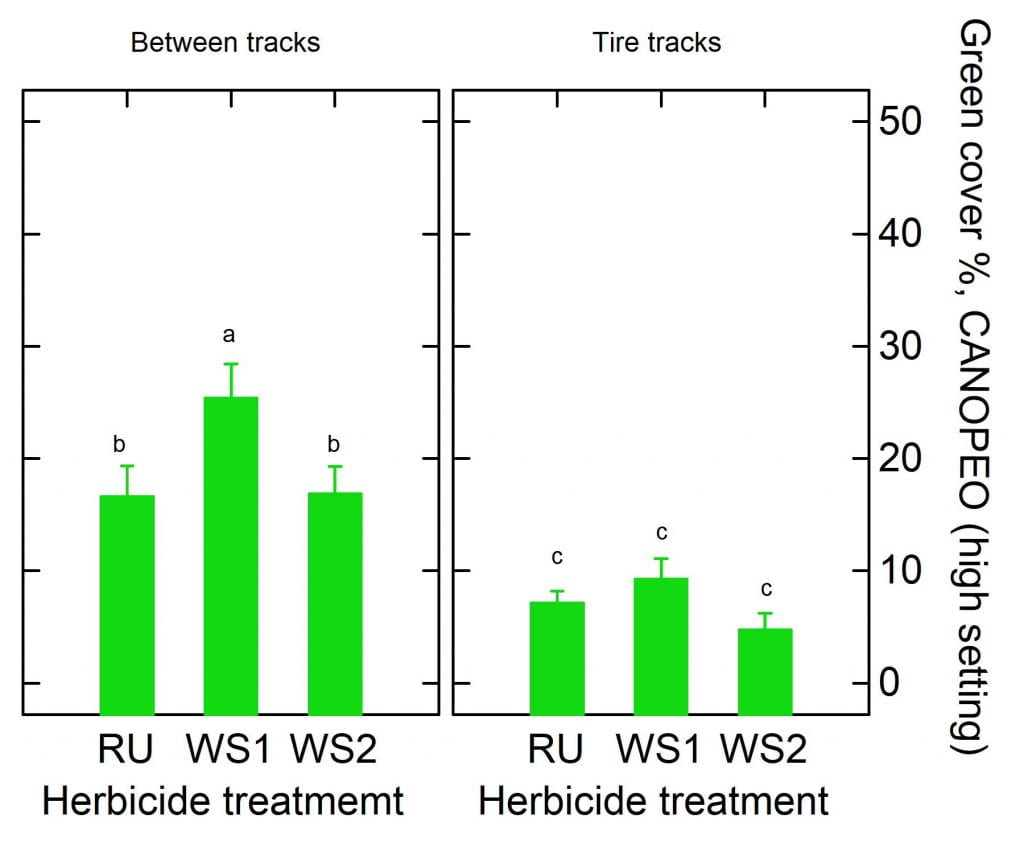
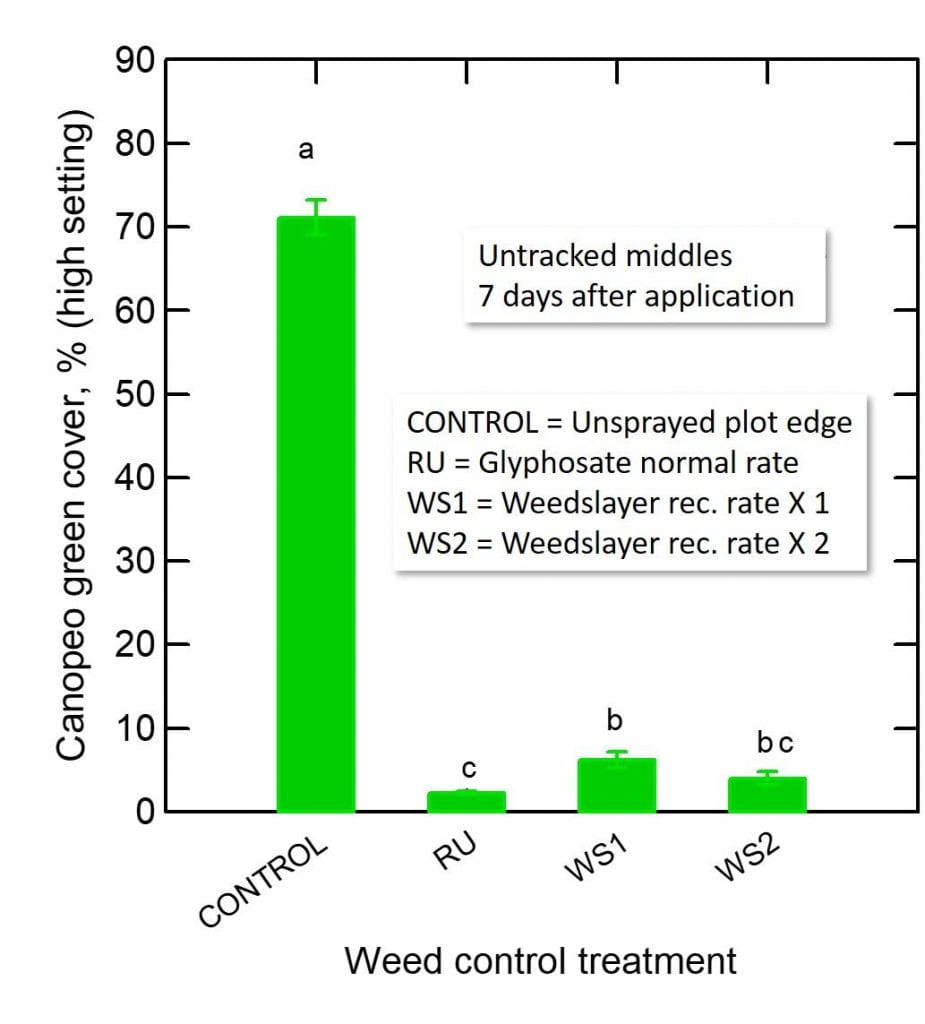
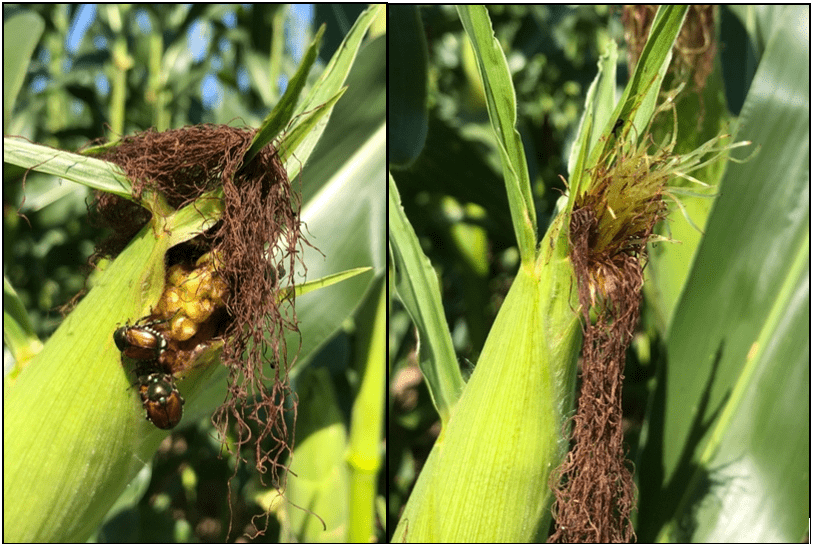
Cerruti R2 Hooks$ and Anahí Espíndola*
$Associate Professor and Extension Specialist, CMNS, Department of Entomology
*Assistant Professor, CMNS, Department of Entomology. Twitter: @Analyssi
Note: This is the seventh and final article of our series on pollinators. Initial articles can be found in the vegetable and fruit headline news June, July and August special editions and Maryland Agronomy News Blog.
Introduction
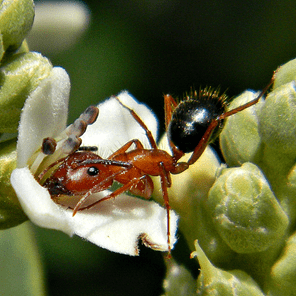
Among insects, wasps (Hymenoptera) which consist of social, parasitic and solitary species exhibit great variations in structure, physiology and behavior (Fig. 1). Further, due to their important roles in ecosystem functioning as pollinators, predators and parasitoids, wasps are studied extensively in agricultural systems and are of specific interest for conservation. There are at least 850 species of social wasps and the vast majority of wasps are solitary (>75,000 species), or parasitic (>650,000 species). Though bees are often mistaken for wasps, a simple way to differentiate them is that wasps contain a pointed lower abdomen and a narrow petiole “waist” that separates the abdomen from the thorax. Also, wasps tend to be less hairy than bees (Figs. 1, 2) and thus are less fuzzy. The lack of hairs does not allow for many pollen grains to attach to their bodies. Consequently, wasps are much less efficient at carrying pollen than their bee relatives. Further, being carnivorous, wasps are interested in flower blossoms mostly as a nectar source, and not as a source of proteins (from pollen). Nevertheless, some wasp species are able pollen vectors, and many play a crucial role as specialist pollinators. Some may be classified as excellent pollinators and in certain systems are much more efficient at pollination than their fuzzy-haired bee cousins. Wasps in one family (Masaridae) have gone through the same dietary transition that bees went through when they evolved to a completely plant-based from a carnivorous diet. Today, this family display dietary similarities with bees, in that they collect and provision larval cells with pollen and nectar, instead of with animal material. In this article, we will demonstrate that wasps do not deserve their “bad guy” image, and that they are more than nuisances or biological control agents. We will show that wasps are fascinating creatures and central to the reproduction of many wild and crop plants.


Food requirements
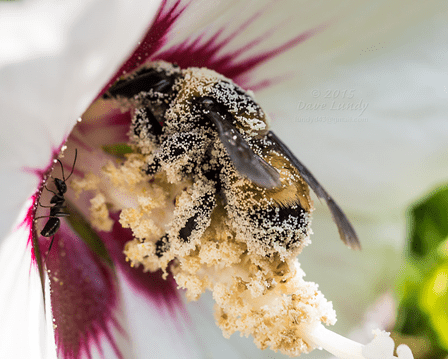
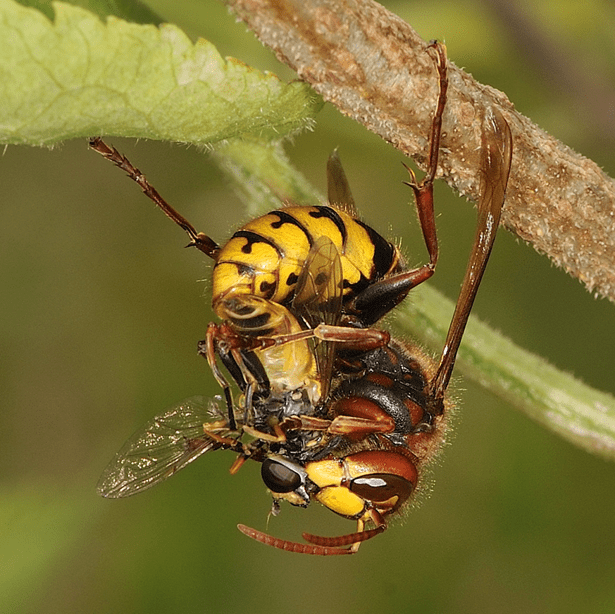
As stated earlier, the vast majority of wasps are carnivorous. However, these wasps cannot survive based on a completely meat-diet; they in fact need to supplement their diets with sugar and water, which very often comes from flower nectar, or honeydew produced by insect herbivores (e.g., aphids). Similar to bees, wasps have high energy requirements, and pollen and nectar allow them to acquire extra energy to use for their high metabolisms. For this same reason, it is not uncommon to observe wasps feeding on fruits (e.g., apples, pears, grapes and blackberries; Fig. 3), fruit juices, honey, jam and pies.

Some wasps, such as yellow jackets, have diverse diets and can consume some of the same foods that humans eat, which is why we often find them swarming our picnic plates and garbage cans. Carnivorous wasps are hunters, and prey on other insects and arthropods, including herbivores that feed on important crops. True wasps have stingers and use them to capture insects or spiders as food for their larvae. Other wasps are parasitic and use their ovipositors to lay eggs on or in the bodies or eggs of other insects, which then become their hosts. In those cases, the immature wasp or developing larva develops inside the host while feeding on its tissues. Many species of parasitic wasps use hosts as direct food or for oviposition. Some female parasitoids feed from and oviposit eggs on the same host individual, while females of other species may use a host solely for food or oviposition. When host feeding occurs, females are believed to obtain nutrients that can be used to produce more eggs, and to enable further host searches.
Importance as pollinators
Wasps are an important part of the flower-visiting guild and often frequent flowers in search of nectar and/or insect prey. Some wasps are considered generalist pollinators, and passively transfer pollen while feeding on nectar from various plants. While doing so, they often overlap with other pollinators, such as bees, flies or butterflies. However, because they generally lack abundant body hairs and do not feed on pollen, they are considered less efficient pollinators than their bee relatives. Further, some behave more frequently as nectar thieves than as true pollinators, especially when they pierce the base of flowers to access the nectar without contacting the plant’s reproductive organs. This said, despite not having the reputation of bees, wasps can and do effectively contribute to pollination. In some plant systems and environments, they can become the most efficient pollinator, surpassing bees. For example, in a study involving pollinators and the plant Schinus terebinthifolius (Fig. 4a), some social wasp pollinators were more abundant and species-rich than bee visitors. Another study found that in some environments, the western yellowjacket (Vespula pensylvanica, Fig. 4b) was a more effective pollinator than the honey bee (Apis mellifera). In that investigation, it was observed that pollen of the plant Scrophularia californica was more efficiently transferred by Vespula wasps and Bombus bees (bumblebees) than by honey bees, which visited the plant but did not pollinate. In this same study, the median number of pollen grains delivered per individual floral visitor also varied among the groups (Apis = 4, Bombus = 9, and Vespula = 34). As a result, this study demonstrated that even though honey bees seemed to be the most abundant floral visitor, the western yellowjacket was the most effective pollinator. Though wasps are sometimes the best pollinator of some generalist flowers, they are typically recognized as specialist pollinators. Specialist unlike generalist pollinators, are very selective in their floral choices, and frequent flowers of one or a very few plant species. In instances where this type of specialization has evolved, rewards involved are either special (e.g., brood site) or inexistent in that the wasp is lured and exploited by the plant. In either case, the plant reproduction relies exclusively on these specialized visitors.

Wasp flowers
Generalist flowers that attract wasps have specific floral traits, such as dull coloration, unusual odor, and readily accessible (exposed) and concentrated nectar. Most wasp can see UV light, and tend to visit white- or yellow-colored flowers such as those in the parsley (Apiacea) family. The majority of wasps have very short mouthparts or tongues, and as such, can obtain nectar only from shallow flowers. Wasp-pollinated flowers can produce either large or small amounts of nectar. One of the plants that are usually visited by wasps is in the orchid family. This plant is pollinated in part or exclusively by social wasps, and is known to secrete abundant nectar, accessible in shallow floral depressions or short spurs. These “wasp” orchid plants also display dull-colors and strongly scented flowers, and are usually avoided by other flower-frequenting insects.
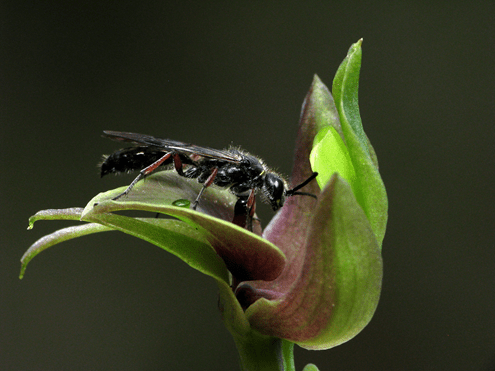
The complexity of floral morphology is usually directly related to their level of pollination specialization. In fact, this complex morphology allows plants to limit nectar availability to one or a few pollinator species that have the “right” matching morphology that allows them to reach the nectar reward. Other traits that plants can evolve are specific odors, which are only perceived by and subsequently attract specialized group of pollinators. It is believed that floral scents play a key role as a selective attractant in numerous specialized pollination systems involving plants and wasps. This is the case of two African pineapple lilies of genus Eucomis. A study showed that two of these plant species – E. autumnalis and E. comosa – are involved in a specialized pollination system with spider wasps (family Pompilidae) in the genus Hemipepsis. Even though flowers of these plants display cryptic coloration (pale yellow-green), the flowers produce strong scents that are only exceptionally attractive to the main pollinators (i.e., spider wasps). Similarly, while the African milkweed Pachycarpus grandifloras (Fig. 5a) has open flowers with abundant and concentrated nectar, they are visited and pollinated almost exclusively by Hemipepsis spider‐hunting wasps (Fig. 5b). In this plant species, it was determined that the achieved specialization with Hemipepsis wasps was facilitated by the production of unpalatable nectar, cryptic coloring and scent, all of which are strong attractants to the pollinating wasps. Though very effective in specialized systems, floral scents are successfully used to attract social wasps in the genus Vespula to the pale yellow-green orchid Coelogyne fimbriata.

Special relationship with orchid – Wasps are well known for their specialized pollination relationship with orchids, with at least 100 species of orchids solely dependent on wasps for pollination. Among these, pollination by sexual deception may be among the most fascinating specialized pollination systems that exist. In many instances, the attractant for pollinators is the orchid’s mimicry of members of the opposite sex. Relative to this, many orchid species display morphologies that mimic the female wasp, thus attracting and tricking male wasps. How they do this? In most cases, it has been shown that these orchids mimic the general appearance and chemical scent of female wasps of specific wasp species. When a male wasp attempts to mate with the “female” (the orchid labellum), it receives and then transfers pollen it collected in a previous floral visit. Sexually-deceptive orchids exist in many parts of the world, with the Mediterranean and Australia recognized as epicenters of sexually-deceptive pollination systems. In Australia, the vast majority of sexually-deceptive orchids are pollinated by thynnine wasps, which patrol areas in search of wingless female wasps to mate with (Fig. 6). In Europe, the most impressive case of sexual-deception is that of species in the orchid genus Ophrys, which have evolved specific odor cocktails to attract different sphecid wasps. Other orchids have been shown to use non-sexual mimicry to attract wasps. A recent study found that two European orchids (Epipactis helleborine and E. purpurata) have developed a chemical-mimicry system in which their flowers release green-leaf volatiles to attract vespid pollinators (Vespula germanica and V. vulgaris). Green-leaf volatiles are typically produced by plants when they are being fed on by herbivores such as caterpillars, and are used by vespid wasps as a cue to find caterpillar prey. Orchids have exploited this cue and evolved the ability to produce these volatiles to specifically lure vespid pollinators into visiting and pollinating their flowers. Thus, orchids are sneaky and have evolved complex ways to attract pollinators, using a mixture of physiological, morphological and chemical deception.
Pompilid wasps
Though this article is meant to serve as a general synopsis of wasp pollination, it is unmanageable to talk about pompilid wasps and pollination (briefly conferred earlier) without commenting at least momentarily on these wasp’s spider hunting behavior (Fig. 5b). Certainly, adult spider wasps are commonly found on flowers. Howbeit, they are also frequently situated on the ground or hovering above in search of prey, specifically spiders. Thus, spider wasps are known more for their spider hunting than their pollination skills. Spider wasps capture spiders by paralyzing them with a sting. They sometimes kill the spider almost instantly and in other occurrences, the spider may survive for a few weeks. Once the spider is paralyzed, a female pompilid makes a wasp’s nest (burrow) or flies or drags the spider to a previously made burrow. A single wasp egg is placed on the spider. Once the egg hatches, the paralyzed spider is eaten alive. Some pompilid wasps are kleptoparasites of other pompilids. Basically, they steal the spider prey caught by other pompilid species. These wasps use one of two strategies. They locate the burrow of the host spider wasp, eat the host spider’s egg and then lay their own egg on the spider. The other strategy is to lay an egg on the spider paralyzed by the host wasp during an inattentive moment. The egg of the kleptoparasitic wasp hatches before the egg of the host wasp, which it subsequently eats. In addition to spider wasps, there are two other “unique” wasp pollinator families that warrant the spotlight: fig wasps (family Agaonidae) and pollen wasps (subfamily Masarinae).
Fig wasps
Fig plants and fig wasps have been evolving together for more than 60 million years, and they depend on each other for reproduction. Each fig is formed by a large number of tiny flowers that face the inside of the fig. When female flowers inside the immature fruit are ready for pollination, the fig emits an enticing aroma that attracts only female fig wasps of a species that specifically pollinate that plant species. After locating the fig, the female pushes herself into the fig through the small opening at the end of the fig. The passage is so tight, that the wasp usually loses her wings and pieces of her antennae. She moves around the fruit’s interior visiting many flowers, laying her eggs inside future seeds that will nourish her progeny while spreading pollen collected from the fig where she was born. After laying her eggs, the female wasp dies inside the fig. Once newborns hatch, they mate and generally, the male chews an escape tunnel out of the fig for the females and then dies. The newly-born females collect pollen, and then exit through the tunnel, searching for a new fig tree to lay their eggs, and restarting the cycle. This complex pollen transfer allows all seeds in the fig fruit to grow (these are the crunchy grains in figs that we eat), while providing a safe place for the wasp’s offspring to develop. However, some fig wasps cheat the plant. Instead of entering the fig and pollinating the flowers, these cheaters use their oviposition appendage to pierce through the fig’s green to purplish skin, and inject their own eggs directly into the fig female flowers, exploiting the pollination service of other female wasps that entered the fig to pollinate, without investing energy and doing the work to pollinate the fig. Other non-pollinating fig wasps include gallers, inquilines (animal exploiting the living space of another), parasitoids of fig pollinators and parasitoids of non-pollinating fig wasps. Some of these wasps may directly attack fig wasps inside the fig and eat the larvae or their food (ovary), contributing to the mortality of fig wasp pollinators.
Pollen wasps
Among wasps, the Masarinae or pollen wasps are a peculiar subfamily of Vespidae (Fig. 7). Unlike their vespid wasp cousins, they exhibit the bee-like habit of provisioning each larval brood cell with pollen and nectar. Females of these wasps use their mouthparts to gather pollen and nectar from flowers and for nest construction. Because pollen wasps feed solely on nectar and pollen, they are also known as the “vegetarian” wasps. Relative to this, it is the only wasp group that provisions brood cells with pollen and nectar rather than insects. There are 300 species of pollen wasp across the globe, and the only regions they have yet to be found include the tropics and Antarctica. Even though there is some morphological diversity in the group, most species are brown and black, with contrasting patterns of white, red and yellow.

Pollen wasps are unique among wasps, not only because of their diets, but also because of their behavior. In fact, many of their characteristics resemble bees. For instance, like most bees, they are solitary and have long mouthparts, which allows them to reach the nectar of flowers with narrow and deep corolla tubes such as beardtongues (Penstemon). However, unlike many bees who display a corbicula (pollen basket) on their hind legs to transport pollen, pollen wasps collect pollen in their crop (expanded portion of their digestive track that can be used to temporarily store pollen and nectar). Like many bees and wasps, it is the females who are responsible for building the nest, which is hard, made of mud and cemented with secretions from their salivary glands. Nests are usually built in concealed places such as under rocks and crevices, although they may be attached to rocks, ledges, and tree twigs. Similar to many bees, each cell in the nest contains a single pollen-nectar loaf on which an egg is laid, and is sealed with a mud plug. Pollen wasps are known to specialize in foraging on very specific flowers, including beardtongues, borage and tansies, in which they play an important role as pollinators. For instance, a number of rare beardtongue species rely on Pseudomasaris vespoides pollination, giving the insect an important role in maintaining ecological diversity.
Natural pest control
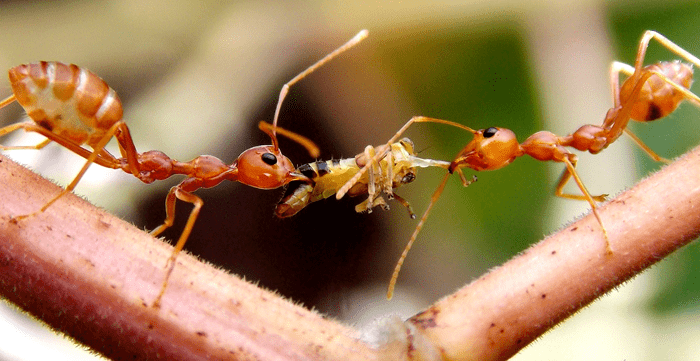
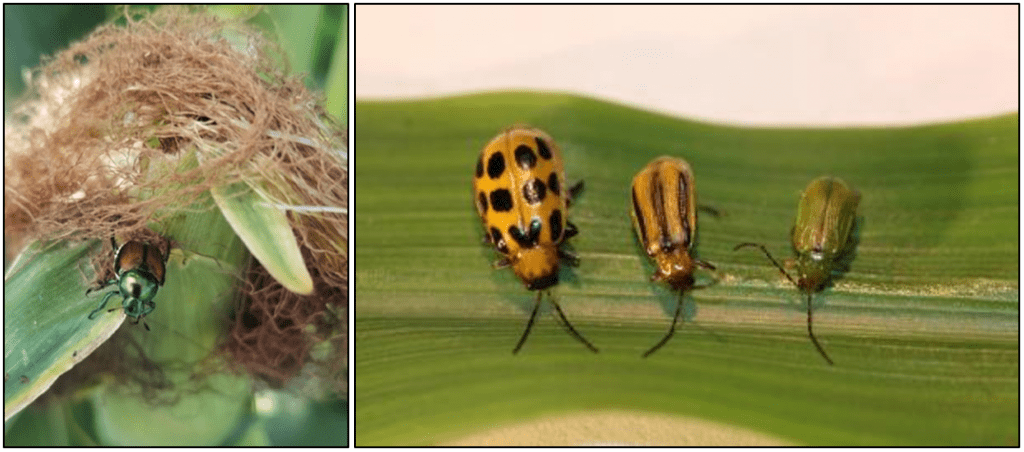
Adult wasps only feed on sugars, which they may obtain by feeding on nectar, honeydew from insects such as aphids, and fruits. However, most wasp species hunt other invertebrates to feed their offspring. Thus, adult wasps do not eat the prey they hunt as they feed it to their young. Despite the fear they sometimes evoke, wasps are extremely beneficial to humans. While searching for nectar, wasps become accidental pollinators, carrying pollen while they travel from plant to plant. However, wasps also serve humans by helping regulate insect pest populations. Relative to this, nearly every insect pest on Earth is preyed upon by a wasp species, either for food or as a host for its larvae. Wasps are so adept at controlling pest populations that the agriculture industry now regularly deploys them to protect crops. A study conducted in Brazil found that social wasps are effective predators that can manage pests in maize and sugarcane. To this point, predatory and parasitic wasps are very valuable natural control agents that help to control insect pest populations in multiple cropping systems. A caveat to this is that one must consider that predatory wasps are generalist predators and, as such, may hunt other beneficial insects such as pollinators, parasitoids, and other predators that may also help control insect pest populations (Fig. 8).
Summary
Wasps do not garner much notoriety as pollinators and are often overshadowed by their bee cousins. Still there are those occasions where wasps are more valuable and efficient pollinators than bees. Additionally, among wasp species are some of the most unique and fascinating pollinators, who sometimes share an intimate relationship with their favorite plants (i.e., orchids). Along this line, are the tiny pollinators of figs who spend most of their life inside a fruit. Without these tiny wasps, many fig varieties commonly consumed may not produce fruits. Further, one should not forget the infamous spider wasps from the family Pompilidae. These wasps are well-known for their viciousness, and ability to subdue spiders much larger than themselves to feed their young (even tarantulas are fearful of spider wasps!). Yet, some of these spider hunting wasps are specialist pollinators of flowers. Though overall, wasps may not be recognized as pollinators, wasp predators and parasitoids have a great reputation for controlling insect pests in multiple cropping systems. For this service, they have earned the praise of farmers and biological control specialists.
Financial support for the publication of this article is via USDA NIFA EIPM grant award numbers 2017-70006-27171