Cerruti R2 Hooks$ and Anahí Espíndola*
$Associate Professor and Extension Specialist, CMNS, Department of Entomology
*Assistant Professor, CMNS, Department of Entomology. Twitter: @Analyssi
Note: This is the eighth article of our series on pollinators. Initial articles can be found in the vegetable and fruit headline news June, July, August and September 2020 special editions and Maryland Agronomy News Blog.
Introduction
There are multiple species and groups of pollinators; and those capable of going airborne are generally more efficient at transferring pollen. Among which, flying insects (e.g., bees, butterflies), birds and bats are the most common, well recognized and intensively studied. Howbeit, many non-flying mammals are also important pollinators; and research in multiple continents (Australia, Africa, South America, etc.) has shown that non-flying mammals (i.e., marsupials, primates and rodents) visit and are quite successful at pollinating flowers. They tend to be more ubiquitous in the tropics where they help pollinate large trees and also assist in the dissemination of their fruits.
Therophily or pollination by non-flying mammals (NFMs) was first described in the 1930s; and worldwide these pollinators have been reported to visit at least 85 plant species. Incidentally, therophilous pollinators were characterized as NFMs mainly to distinguish them from bats. Arguably, the best examples of this pollinator group are marsupials (e.g., honey possum) prevalent in Australia, and primates (e.g., monkeys, lemurs) found in territories such as Madagascar. Funnily enough, of the plenteous interactions between plants and their animal pollinators, one of the least foreseeable surprises is represented by rodents (e.g., mice, rats, gerbils, squirrels). Who would have thought that among rodents’ duties are delivering pollen to plants? Yet, rodents are important pollen vectors of many plants. The first description of these animals as pollinators date from the early 1970s in South Africa, where they were observed to pollinate a shrub from the sugarbush genus Protea (Proteaceae, Fig. 1a, b). Since then, they have been reported pollinating other plant families (e.g., Colchicaceae, Cytinaceae, Fabaceae, Melastomataceae, Hyacinthaceae and Ericaceae). Despite these interesting observations, not much research has been directed at determining whether or not flowers are indeed pollinated by these rodents. This article will summarize some known facts about NFM pollinators with a greater emphasis on rodents.
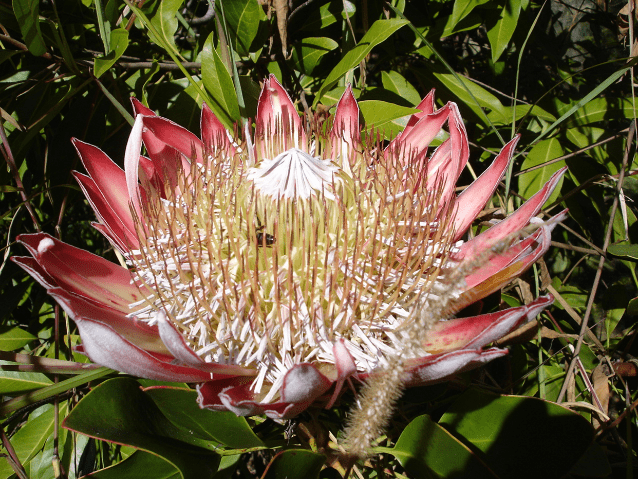
General floral traits associated with Non-Flying Mammal pollinators
Because plants adapted to pollination by NFMs tend to converge in their morphologies (what is called a “pollination syndrome”), it is generally possible to predict whether plants are pollinated by NFMs by evaluating their floral characteristics. Indeed, plants adapted to pollination by NFMs display some unique vegetative and floral traits that helps protect their valuable pollen and nectar from birds, insects and other non-therophilous pollinators. These flowers are generally large and robust, which is thought to be an acclamation that allows them to withstand the teeth of hungry and aggressive feeders, and are typically arranged as multi-flower inflorescences. Contrasting those pollinated by birds, flowers pollinated by NFMs are often drab in color, but exude a pungent odor sometimes described as musty or yeasty, which assists in their discovery by mammals (e.g., rodents and shrews) that rely mostly on their sense of smell. Flowers generally contain protruded styles and stamens, an abundance of sugar-rich nectar and large amounts of pollen. This structure allows for increased precision in pollen delivery and transfer by NFMs, whom because of their large size are clumsy flower handlers. Some additional clues that suggest plants are adapted to pollination by NFMs include: i) evidence of nondestructive feeding on flower; ii) infrequent insect or bird visitation, and iii) nectar secretion, scent production and floral anthesis synchronized with the most active periods of the interacting NFMs.
Pollination syndromes are specific combinations of floral traits having evolved in adaptation to special pollinator guilds; and traits listed in the aforesaid paragraph represent the pollination syndromes of NFMs. However, NFMs have varying morphologies and foraging behaviors, suggesting that floral features may still differ according to the specific pollinator species within this non-flying guild. For instance, the ability of different species to pollinate a flower may vary according to their size, mode of locomotion, frequency of visitation and constancy to a particular plant species. Non-flying mammals can range in size from miniscule (pygmy possum) to large (monkey) creatures, and their foraging habits differ between nocturnal (e.g., marsupials and most rodents) and diurnal (e.g., primates). Regarding floral features, primate pollinated flowers tend to be unscented and very large, marsupial pollinated flowers are typically in the canopy, and flowers pollinated by rodents are generally at ground level and can be smelly. Further, many floral features associated with the NFM pollination syndrome (e.g., copious amounts of sugar-rich nectar) can be shared with those present in other pollination syndromes, such as those associated with insects and bats. It is for this reason that flowers once thought to be pollinated by bats based on their morphology were eventually discovered to be pollinated by NFMs. This is the case of Mucuna birdwoodiana, which was determined to be primarily pollinated by the masked palm civets, Paguma larvata and the Pallas’s squirrel (aka red-bellied tree squirrel, Callosciurus erythraeus styani, Fig. 2).

Primate and marsupial pollination

Some of the best-known groups of NFM pollinators are those formed by the primates of Madagascar and South America, and the marsupials of Australia. Primate nectar feeding and cross-pollination of flowers was not widely known in the past but is well acknowledged today; and there may be more species of primates involved in pollination than any other group. Specifically, on the island of Madagascar, there are some unique primate pollinators. Some of which include the red-bellied (Eulemur rubriventer, Fig. 3) and black-and-white ruffed (Varecia variegate, Fig. 4) lemurs. The red-bellied lemur has a brush-shaped tongue that helps it effortlessly forage nectar from flowers, and indirectly pollinate Vahimberona and guava. This lemur has been observed browsing on flowers and leaves of over 70 plant species (e.g., the traveler’s palm Ravenala), which makes them also potential pollinators of those plants as well. Interestingly, they also feed at night on eucalyptus flowers, whose flowers are nocturnal. The black-and-white ruffed lemur, which also resides in Madagascar, is the world’s largest pollinator and main pollinator of traveler’s palm. For this, they are uniquely equipped to open their flowers, using their prehensile hands to pull open the tough flower bracts and then stick their long snouts and tongues inside to feed on the nectar. While doing so, pollen sticks to their muzzle and fur, and is subsequently transferred to the next flower where they repeat these motions. Because they feed on fruits, lemurs contribute to plant reproduction and survival also through seed-dispersal, passing viable fruit seeds in their feces. Another unique primate pollinator is the bush baby (galagos) which are among the smallest primates. Bush babies have very large eyes which are advantageous to their nocturnal habits. These tiny primates feed on the white flowers of baobab (Adansonia) trees, eating parts of the flower and, while doing so, transferring pollen. In addition to flowers, bush babies feed on insects, acacia gum, seeds, bird eggs and fruits.

Small marsupials tend to be nocturnal and cryptic, which makes it an arduous task to monitor them directly. Speaking of small, among marsupial pollinators is the tiny honey possum (Tarsipes rostratus, Fig. 5) of Australia which ranges in mass from 6 to 18 g (0.2 to 0.6 oz) and has a body length of 60 to 90 mm (2.4 to 3.5 in). Honey possums devour pollen and nectar from a variety of flowering plants. Large amounts are consumed from plants belonging to the families Proteaceae, Epacridaceae and Myrtacae. They are the only flightless animal that feeds exclusively on pollen and nectar. Honey possums are adapted morphologically and physiologically to their unique nectar and pollen diet. As such, they have several physical features handy for pollination including grasping feet and a prehensile tail used to wrap around tree branches, which allow them to hang while searching for flowers. It also has an extremely long and specialized extensible tongue, which has a brush-like tip adapted to gathering pollen and nectar. While feeding, its long-pointed snout gets dusted with pollen, which can then be transferred to a different flower. These tiny marsupials are known to pollinate the very specious Australian plant genera Banksia and Eucalyptus.

Another marsupial pollinator is the sugar glider (Petaurus breviceps), which gliding technique has been compared to that of flying squirrels. Sugar gliders have a flexible diet that may vary according to location and season. For this reason, in addition to nectar and pollen, they feed on plant sap and gum (e.g., from Acacia and Eucalyptus), spiders, small birds, insects and the honeydew secretion of sap-sucking insects. Sugar gliders are important pollinators of native Australian flowering plants. Another glider, the yellow-bellied glider (Petaurus australis) feeds on the nectar of Banksia and Eucalyptus, and their diet consists largely of nectar, pollen and the sap of eucalypts.
Rodent pollination
Multiple species of gerbils, mice, rats and shrews visit flowers, and as they move into a flower to feed on nectar, their heads get dusted in pollen, which can then be transferred to other flowers. Generally, several plant features are linked to the rodent pollination syndrome. Among these traits are robust dull-colored flowers (lack colorful petals that attract insects or birds), yeasty or musty scent, flowering in the winter-spring, the presence of stiff reproductive organs, and easy access to nectar. Inflorescences of plants pollinated by rodents may be hidden deep inside the branches (e.g., Protea nana, P. cordata, Leucospermum arenarium) and are often at or near ground level where they are readily accessible to rodents (aka geoflory). This latter trait is indeed one of the most-commonly present traits in rodent-pollinated plants. Additional features in these rodent-pollinated flowers are abundant pollen, and easily-accessible and copious nectar secreted in the evening and during winter flowering. The latter characteristic is understood to serve the large caloric needs of small mammals, especially during the food-scarce winter time. Therophilous plants that flower in spring are recognized to provide nectar during rodents’ breeding period, when caloric intake can be a major limiting factor. Because of their abundant pollen and nectar, rodent flowers are a popular commodity to rodents due to them being sources of protein (pollen) and sugars (nectar). A great example of a plant being adapted to fulfil the nutritional requirements of their rodent pollinators is certain species of Protea. These flowers present copious amounts of xylose sugars in their nectars, which is rejected by birds but is uniquely adapted to the nutritional abilities of their specialized rodents, the spiny mice. These plant species are not only good nutritional sources for the spiny mice, but they also provide those resources during a period when other food sources are scarce. From this respect, these plants are specifically adapted to allowing the subsistence of these very specific mammal pollinator.
Plant/floral features associated with the rodent pollination syndrome are often used to accurately determine whether plants are rodent-pollinated. For example, the Pagoda lily, Whiteheadia bifolia was hypothesized to be pollinated by rodents on the basis of containing most features listed in the aforesaid paragraph. Later, a study confirmed that the Namaqua Rock Mouse (Aethomys namaquensis, Fig. 6a, b) uses their snout to transfer pollen between Pagoda lily plants. The Pagoda lily period of winter flowering was shown to correspond with a time of food shortage and breeding season of the Namaqua rock mouse. Its flowers are close to the ground and thus reachable by mice. The plant is very robust, especially the stamens, such that they are not damaged during mice visitation. It is not conspicuously colored making it unnoticeable to other pollinators, but which is also adapted to the nocturnal activity of the Namaqua rock mouse.

Though colorless flowers are a well-known trait of the rodent floral syndrome, color plays a role in attracting rodents in some cases. For example, the Chinese orchid, Cymbidium serratum which is pollinated by wild mountain mice, Rattus fulvescens uses color and odor as attractants. The smell is however, stronger at night. Similar to the Pagoda lily, the Chinese orchid flowers when mice are active and other food resources are in short supply, which occurs in early spring. Notwithstanding, uncharacteristic of this rodent pollinated flower, the Chinese orchid doesn’t contain nectar. The food reward is its labellum which is slightly sweet while other flower parts are bitter. While feeding on the labellum, the mountain mouse comes in contact with stigmas and pollinia.

Evidence for rodent pollination
Although it is becoming more widely-accepted that rodents are important pollinators, the fact that they are mostly nocturnal poses a challenge in observing their behavior and suggests that the contribution of rodents to plant pollination is currently underestimated. A clear sign that a rodent is indeed involved in a pollination interaction with their preferred plant(s) can be obtained if pollen is found in their feces (ingested through the process of grooming). On this, a study found the hedgehog lily, Massonia depressa pollen on snouts and in the feces of captured rodents. Another clear test for rodent pollination is the observed reduction of seed production in flowers experimentally excluded from rodents. This has been shown in plants such as Leucospermum arenarium.
In some cases, rodents work in concert with other pollinator groups. An example of this is that of hummingbirds and rodents pollinating Meriania sanguinea. Another fascinating case is the one of the chestnut spiny rat (Niviventer fulvescens) and short-nosed fruit bat (Cynopterus sphinx) pollinating Mucuna championii through “explosive” flower openings. Specifically, flowers of these plants remain closed, with their reproductive organs tightly packed within the closed flower. Once a sufficiently-heavy pollinator (e.g., rat, bat) reaches the flower and tries to access its nectar, they trigger with their weight, the release of the pressure built in the flower, which leads to an explosive opening. Through this, the pollen which was tightly packed in the flower prior to the explosion is expelled and lands directly on the pollinator’s face, which transfers it to a different flower during a future flower visit.
Are there any benefits to invasive rodent pollinators?

Non-native rodents have invaded about 80% of the world’s islands, posing a severe threat to native insular biodiversity. Though invasive species can be disruptive, by feeding on or competing with native organisms and severely disrupting biodiversity; in some instances, it has been suggested that invasive rodents may fulfill pollination niches vacated by endemic pollinators which may have become extinct or threatened because of their invasion. A study conducted in New Zealand suggested that the invasive black rat (aka ship rat, Rattus rattus) partly maintained pollination of three forest plant species, which without this compensation would be currently significantly more pollen-limited. Thus, while the ship rat is known to have a negative impact on biodiversity overall, evidence showed that it contributed to a crucial ecosystem function (i.e., pollination) for some plants on some of the invaded islands. Likewise, another study examining the potential of the same alien black rat (Fig. 7) to pollinate the Australian native plant Banksia ericifolia (Fig. 8), found that although black rats frequently visit B. ericifolia, they were unable to properly compensate for the pollination loss associated to the extinction of their native mammal pollinator. Though these findings suggest that invasive rats may remediate in some cases the loss of pollination associated to the invasion events, many investigations demonstrate that their destruction outweighs their benefits on these islands. This is due to their strong negative effects on other aspects of the native biodiversity and human well-being.

Summary
Non-flying mammals do not garner much notoriety and excitement as pollinators, and are often overshadowed by showy flying pollinators such as bats, birds and insects (e.g., bees, moths and butterflies). This may be partially due to the fact that these non-flying pollinators are often nocturnal and cryptic, which may cause their contribution to pollination to be underestimated. Despite this, increasing numbers of studies indicate that NFM pollinators are important for dispersing pollen and fruits, and that multiple family of plants have vegetative and floral traits adapted specifically to their pollination. Non-flying mammal pollinators are represented by three main groups: primates, marsupials and rodents. Because of their large size and energy requirements, plants pollinated by these pollinators often contain copious amounts of nectar and pollen. Most plant species pollinated by these animals are present in Africa, South America, South East Asia and Australia. Among all these species, the plant family Proteaceae was the first reported NFM pollinated plant family and is one of the most studied to date. However, with the increase in awareness of the existence of these pollinators, new examples appear regularly, demonstrating that their contribution to pollination may be more common than initially thought. Finally, recent works have shown that invasive rodents can be extremely detrimental to most biodiversity processes on islands and are capable of causing the extinction of native pollinators. Notwithstanding, some invasive rodents are capable of partially compensating for lost pollination services associated with the extinction of native pollinators.
Financial support for the publication of this article is via USDA NIFA EIPM grant award numbers 2017-70006-27171.




