Cerruti R2 Hooks$ and Anahí Espíndola*
$Associate Professor and Extension Specialist, CMNS, Department of Entomology
*Assistant Professor, CMNS, Department of Entomology. Twitter: @Analyssi
Note: This is the third article of our series on pollinators. The initial articles can be found at the vegetable and fruit headlines news June special editions and Maryland Agronomy News Blog.
Introduction
Much has been written about bee (Hymenoptera) and butterfly (Lepidoptera) pollinators. Flies (Diptera) have also gotten their fair share of press regarding their pollination services. However, beetles (Coleoptera), which represent the largest insect order and are among the first flower-visiting insects in history, don’t receive similar admiration from pollinator enthusiasts and paparazzi. Nevertheless, even now, beetles are considered essential pollinators; and are especially important pollinators of some of the first flowering plants to evolve, such as magnolias and spicebush (Fig. 1). Beetles are attracted mostly to flowers that emit musky, yeasty, spicy, rotten or fermented odors. It just so happens that spicebush and magnolia flowers contain spicy pollen and produce aromatic oils, respectively, each of which serves as a lure for their beetle pollinators.

There are 380,000 named living species of beetles, constituting nearly one-fourth of all known animal species on our planet. Beetles are so abundant that there are more species of beetles than of any other known group of animals. Among insects, beetles make up about 40% of all known species, and this may be due to their amazing ecological diversity, which allowed them to evolve impressive diverse morphological features. In the context of pollination, due to their sheer numbers, beetles contribute to a considerable amount of plant pollination. Even though the most consistent pollinators today belong to other insect orders, beetles do contribute significantly to the pollination of specific plants. Further, because beetles were one of the first insects to establish pollen-based relationships with plants, they have been described as key contributors to the reproduction of the first groups of flowering plant species.
Beetle history as pollinators
Beetles have been a predominant group of floral visitors and pollinators since very early times in the evolution of plants. Beetles have been evolving for roughly 250 million years, and are recognized among the first insects acting as pollinators of flowering plants. Indeed, until recently, many beetles and other insects were identified as pollinators of non-flowering plants (e.g. conifers, ginkgos, cycads). However, many ancient fossils indicate that plants today that are mostly wind-pollinated, relied strongly on insects for pollination in the past. Interestingly, a very recent study of fossil beetles found in amber (like the fossil insects from Jurassic Park!) provided the oldest account of flowering plant pollination. In this study, four different species of extinct beetles having lived 99 million years ago were found to carry pollen of flowering and non-flowering plants. While the non-flowering plant pollen corresponded to different species of cycads, pollen from flowering plants belonged to a newly described species of water lilies (Fig. 2). This not only pushed back the time of the first known fossil record of pollination, it also represents a clear link of how pollination of flowering plants may have evolved from pollinators that were visiting non-flowering plants. In comparison to other insect species currently known to visit flowers, beetles are now the oldest known pollinators of flowering plants, predating the oldest pollen-rich bee fossil by roughly 30 million years.

Modern-day beetle pollinators visit many different types of plants, feeding on pollen, floral parts, and sometimes on nectar. While most beetles that act as pollinators are not specialized in their floral choices, beetles that visit flowers have some morphological traits that make them relatively efficient in transferring pollen. In fact, many of these beetles are hairy, allowing for pollen grains to stick on them and be transferred among plants. Further, some beetle groups have modified mandibles, with a brush-like structure or elongated proboscis, which allows them to more readily collect pollen and feed on nectar, respectively. While the vast majority of generalist beetle species visit well known plants such as carrot, buttercup, sunflower or cabbage, some beetle species are specialist pollinators of descendants of plants they visited millions of years ago: water lilies, custard apples, magnolias, all spice plants. For these latter plants, it is suspected that the evolution of beetles that they are associated with was driven by the diversification of their host plants. Relative to this point, part of the amazing morphological diversity that we see in extant beetle species is due to their long-dated interaction with these plants. Further, because these latter plant groups are usually associated with tropical or Mediterranean environments, it has been assumed for a long time that beetle pollination is most important in warm climates.
Beetles and magnolia’s special bond
Pollination by beetles seems to have strongly influenced the evolution of angiosperm flowers. Many million years ago, plants and pollinators began specializing and adapting traits that benefited each other’s requirements; and beetles were among the earliest pollinators to take part in nature’s matching adventures. Thus, flowers of lineages such as magnolias are paired with beetles as their primary pollinators (Fig. 3). Today, this flower’s characteristics are recognized as those that make up a “typical” beetle flower. These flowers are bowl-shaped and provide a cave-like structure for beetles to use as shelter. Beetles pollinating these flowers feed on pollen and sweet floral secretions. Though Magnolias from some areas are pollinated by several beetle families, some Magnolias from tropical areas have very specialized interactions, and are only effectively pollinated by few beetle species.

But how do beetles pollinate Magnolias? The story goes that beetles enter the flower carrying pollen from a previous floral visit, and passively deposit the pollen onto female structures while roaming about in the flower and feeding on stigmatic secretions. While these visits are happening, pollen of the flower is released, and while beetles feed on it, they also get it all over themselves. At the end of the flowering cycle, the flower wilts, and the messy beetles move on to another wide-open flower, where pollen is transferred and collection from the new flower initiates. This relationship between Magnolias and their beetle pollinators has evolved over millions of years of interactions. For example, magnolia plants have evolved strategies to “direct” beetle feeding away from reproductive parts of the flower. Basically, the plant sacrifices pollen and leaf tissue for the service of pollination. More specifically, beetles feed on other areas of the flower and are thus distracted from feeding on female parts of the flower which needs to stay in tacked so that it can produce seeds. Related to this, it has been suggested that this beetle-pollination relationship has led to an increase in the number of stamens produced by the plant, which insures that some are still present and ready to participate in plant reproduction after beetles finish their feast. Moreover, magnolia has developed securities that ban access by other insects during critical pollination phases and produces a fruity-smell that attracts beetles to the flowers.
Why do beetles visit flowers?
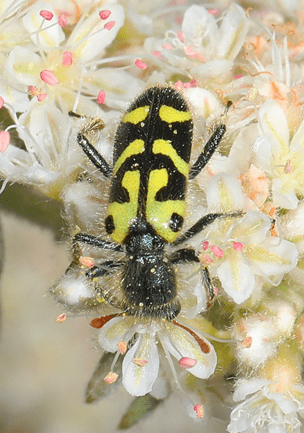
To understand beetle pollination, it is important to realize that most beetles visit flowers to feed on pollen, and sometimes on floral structures (e.g. petals, anthers) or secretions (e.g. stigmatic secretions). In fact, beetles rarely visit flowers for the typical nectar that other famous pollinators seek, and this reward is actually often absent or moderately produced in flowers beetles frequent. The most important reward beetles are after when visiting flowers is protein-rich pollen. Pollen beetles (Nitidulidae), longhorn beetles (Cerambycidae), leaf beetles (Chrysomelidae), rove beetles (Staphylinidae), scarabs (Scarabeidae), tumbling flower beetles (Mordellidae) and weevils (Curculionidae) are common pollen feeders of many flowers. Tumbling flower beetles are commonly found feeding on flowers of the carrot family, which purportedly contain a good pollen source. Though most beetles visit flowers solely for pollen, there are some species that desire more. Many beetle families such as checkered beetles (Cleridae; Fig. 4), and blister beetles (Meloidae) are known to use other floral resources. Indeed, some prefer to simply eat the flowers and will gnaw flower tissue, eat pollen and lick floral exudates. However, in the case of some beetles, flowers represent much more than just a place to get free food. For example, monkey beetles (Scarabaeidae), a primary pollinator of the peacock moraeas plant in South Africa, frequent flowers of this plant for pollen, nectar and mating, while beetles from South America are attracted by heat-producing Aroid plants and a series of water lilies, in which they feed and reproduce. More locally, many beetles, including soldier beetles, often use the same flower that they frequent in search of food for mating (Fig. 5). Besides using flowers as sources of food and mating, beetles sometimes use them as their residence and a place to prey on other insects. For example, predatory beetles often hide within flowers, waiting for soft-bodied insects to visit. Ladybeetles, which are well known predators, visit flowers to feast on aphids and may sip some nectar when available. Beetles may also use flower blooms as a place to hide from predators. Some beetles may also enter flowers because the temperature within their blossom is more preferable to external environmental conditions. Thus, beetle-pollinated flowers include either sufficient nutritional rewards, place to warm up, mate and hunt or serve as a refuge from natural enemies.

Beetle importance as pollinators
Beetles make up the largest group of pollinating animals because of their large numbers and are the most diverse group of pollinators in the US. More than 77,000 beetle species are estimated to visit flowers. Of the world’s almost 350,000 flowering plant species, beetles are believed by some to be responsible for visiting nearly 90%. Cantharophily (cross-pollination of flowers by beetles) is more common in tropical areas especially in tropical-Mediterranean regions and is often not considered important to pollination in temperate regions. Relative to this, the native paw-paws and atemoya are some of the only crops in the U.S. known to be pollinated by beetles. However, an estimated 52 native plant species are pollinated by beetles in North America north of Mexico, and there are several common temperate ornamental plants that are beetle pollinated. Beetles may also be particularly important in semi-desertic areas, such as South Africa and southern California. Beetles are important for the production of crops that are exclusively beetle-pollinated, such as atemoyas or paw-paws. To this point, a relatively recent study found that atemoya orchards in Australia directly benefit from the presence of wild beetle species in surrounding forests, and that increasing the presence of natural habitats around cropping areas improves fruit set and production. From this respect, the presence of wild beetles is an extremely valuable ecosystem service, which if present, can help some industries which rely on hand-pollination cut labor cost.
Despite contributing to the pollination of many plants, beetles’ roles in ecosystem services are generally assumed to revolve around their scavenging and organic matter decomposition. Part of this misconception may be attributable to the fact that flower-visiting beetles are less active on flowers than are many flies, butterflies and bees, and for that reason they are usually considered inefficient pollinators. Further, some scientists claim that beetle pollination is among the most destructive: most beetles eat their way through petals and other flower parts, they defecate within the flower, and then spread the mixture of feces and pollen. Still, beetles are recognized as the primary pollen transporters for numerous plant families, especially phylogenetically basal plants such as magnolias and water lilies. For this reason, it is unfair to not recognize beetles as vital pollinators who play a unique role in wild plant reproduction and food production.
Beetle floral preference
Flower description. Flowers dependent on beetle pollinators contain a foreseeable list of features that differ considerably from flowers primarily pollinated by other insect orders. Flowers visited by beetles may be large solitary (e.g. magnolias, water lilies) or clusters of small flowers (goldenrods, Fig. 6; Spirea, Fig. 7). Beetles are generally clumsy and rough fliers, compared to more delicate and/or agile flying insect pollinators (e.g. butterflies, bees, flies). To accommodate these clumsy fliers, beetle-pollinated flowers tend to be large and their rewards easily accessible. Further, the volume of individual flowers is typically large enough to accommodate several beetles within the same floral cup. Beetle-pollinated flowers usually have open corollas, solitary and heavily-constructed, though some may contain many tiny clustered flowers. Beetles also frequent flat to dish-shaped or bowl-shaped flowers, as these features provide them an easy platform for landing and often a good place for shelter.
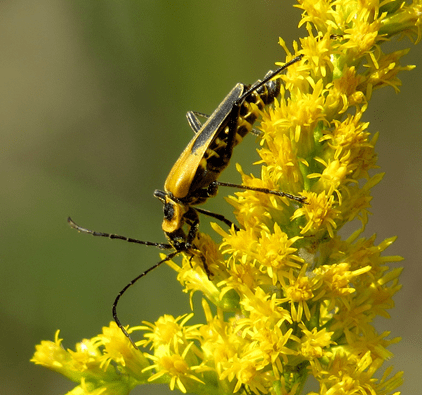
Relatively large beetles can often damage flowers, or their pollinating parts, especially when feeding on pollen with their large mouth-parts. However, many flowers resist this damage either by producing many more flowers than what would be sufficient for reproduction, or by physically protecting the most important parts of their reproductive organs (e.g. the ovaries) from beetles. Some beetle-pollinated flowers such as some Aroids present structures that function as traps, preventing beetles from leaving the flower before the end of the plant reproductive cycle. Others floral traits that prove beneficial to beetle attraction include nocturnal (but also diurnal) blooms, plant heat production, and the presence of floral structures that provide protection to beetles.

Flower color. Although many beetles can see color and UV light, they use color mostly as a short-distance cue for floral choice. Indeed, it seems that most beetles base their long-distance floral location on floral odors. In terms of floral coloration, most beetles are attracted to dull-colored, greenish or white, and reddish brown or dark flowers. Flower size is not a good indicator of floral choice, since flowers preferred by beetles can be large or small and clustered.
Floral odors. Many flowers pollinated exclusively by beetles display strong fragrances. Odors that serve as primary beetle attractants are numerous and not always pleasant, including smells of decaying plant or animal material, fermented fruits or spices. Indeed, when locating flowers, beetles are attracted to a variety of scents and although there are not many plants pollinated primarily by beetles, flowers that do depend on them are typically characterized by the presence of discernible fragrances that acts as a primary long-distance attractant.
Pollen load and heat. Many beetle species eat pollen, so it is understandable that plants they frequent produce plenty of easily-accessible pollen. This also ensures that there is enough pollen that remains for pollinating the flower after the beetles complete their meal. In addition to pollen, beetle flowers often use heat as a reward for pollination. Some incredible plants are capable of producing heat, which attracts and likely increases the activity level of beetles while visiting their flowers. These heat-producing beetle-attracting flowers belong to families Nymphaeaceae, Illiciaceae and Magnoliaceae.
How to Protect beetles?
Planting swaths of wild flowers, native shrubs and trees, as well as urban green spaces will provide good habitats for adult beetles and other pollinators. Similarly, since some beetles deposit their eggs in soil or loose-leaf litter, it is critical to eliminate the use of synthetic fertilizers and toxic pesticides that threaten their life above and below the soil. This is especially important for soldier beetle larvae which are carnivorous, consequently foraging for aphid eggs, worms, slugs, and other prey among assorted plant debris. As they feed, soldier beetle larvae reduce the number of soft-bodied insects, such as aphids. Adopting organic land management practices such as planting pollinator habitat, conservation strips and cover crops, using mulch for weed control, and adding compost and diverse plantings to arable lands, helps to build and protect beetle biodiversity.
Summary
Though currently recognized as being among the oldest known pollinators of flowering plants, beetles usually don’t get their due as major pollinators. This is partially attributable to their suspected inability to move pollen through long distances and their destructive behavior while feeding on pollen and floral structures. Notwithstanding, beetles are central in the pollination of many plant species in temperate areas and are popularly known for their pollination services in tropical and Mediterranean ecosystems. In temperate regions, beetles contribute to the pollination of Magnolias and all spice bushes. In terms of crops, beetles are important in the production of some tropical fruits such as atemoyas and local paw-paws, allowing these crops to be produced without hand-pollination. Pollinating-beetle conservation involves protecting established ecosystems and increasing the presence of wild habitats by establishing natural resources such as wild flowers, native shrubs and trees. Financial support for the publication of this article is via USDA NIFA EIPM grant award numbers 2017-70006-27171.