Much of our understanding about chemistry at the quantum level is obtained through the studies of elementary reactions in the gas phase, a prominent example being H + H2 → H2 + H and its isotopic variants (e.g., H + HD → H2 + D). The simplicity of this system allowed highly accurate theoretical treatments of both the potential energy surface (PES) and the dynamics thereon. Together with sophisticated experiments, studies revealed how the reaction is dictated by principles of quantum mechanics, including the interference between different quantized transition-state pathways, interference between different paths around a conical intersection (CI) , and geometric phase effects.
Ultracold molecules promises to revolutionize the field of reaction dynamics by bringing unprecedented control to how the reactions are initiated. The types of molecules that have currently being brought to ultralow temperatures, however, remains limited to heavier species (e.g., KRb, BaF) with many electrons. Full quantum descriptions of reactions involving these species are challenging even with state-of-the-art theoretical methods.
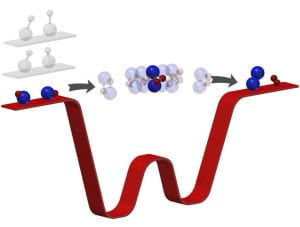
Here in the Liu lab, we initiating a new experiment to explore isotope exchange reactions of Lithium in the ultracold regime, including triatomic reactions such as
6Li7Li + 7Li → 6Li + 7Li2,
and tetratomic reactions such as
6Li7Li + 6Li7Li → 6Li2 + 7Li2.
This system combines theoretical tractability (Li is one of the lightest elements) with experimental control (laser cooling and quantum control of Li is highly mature). On this new platform, we will explore many fascinating topics in the field of chemical reaction dynamics. Several examples are given below.
Coherent control of bi-molecular reactivity
Individual reaction events are, in principle, quantum coherent processes. Can we influence the formation products in various allowed exit channels by controlling the quantum states of the reactants?
Transition between statistical and non-statistical dynamics
What causes the same chemical reaction to take a simple trajectory (non-statistical) across the potential energy surface (PES) in some cases but a complex one (statistical) in others?
Entanglement between reaction products
The products of a chemical reaction exist, in principle, in an entangled superposition of allowed states. Can we detect and harness this entanglement?
Assembly of ultracold clusters
The coherent assembly of pairs of ultracold atoms into diatomic molecules revolutionized the field. Can we apply the same principles to assemble larger clusters (e.g., triatomic, tetratomic, etc.) as to achieve full internal state control for increasingly large quantum objects?