RESEARCH GOALS
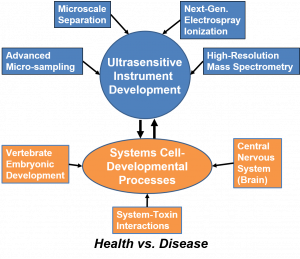
We study how proteins and metabolites control cell fate, tissue patterning, and neural circuit function. We build and refine capillary electrophoresis–MS and liquid chromatography–MS to measure tiny samples with rigor and in ultrahigh sensitivity. We then apply these unique measurement tools in vertebrate development, brain circuits, aging, and addiction to move from molecules to mechanism in space and time. To achieve these goals, our objectives transcend across classical disciplines.
Guiding questions
- Where do proteome and metabolome control points sit relative to genes and phenotype.
- How do single cells and micro-regions change as tissues form and circuits mature.
- Which early molecular changes forecast resilience or vulnerability in aging and addiction.
Capabilities that enable our studies
- Targeted sampling in intact (frog) embryos and brain tissues (mouse, rat). This includes single identified cells, single neurons, and defined micro-regions.
- Orthogonal separations. CE–MS for polar and charged analytes at minute inputs. LC–MS for depth, robustness, and targeted assays.
- Acquisition tuned for scarce material. Rapid single-cell windows. Validated multiplexed quantification. DIA and PRM when appropriate.
- Automation and real-time logic for throughput and reproducibility.
- Independent validation with imaging or physiology when relevant.
- Transparent quality controls and statistics so results are reproducible.
Focus areas
-
Developmental neurobiology and vertebrate embryonic patterning
Problem: Cell fate decisions emerge before transcripts tell the whole story.
Approach: Lineage-guided sampling and quantitative proteo-metabolomics in live vertebrate embryos.
What we learn: Molecular asymmetries along embryonic axes. Small-molecule and protein programs that influence organizer function and neural induction.
Why it matters: These results reveal upstream levers for early patterning and suggest new ways to probe or redirect fate. -
Neural circuits and brain development
Problem: Circuits transition from growth to precision. Molecular timing is key.
Approach: Micro-region proteomics of small brain nuclei. Function-linked single-neuron proteomics after electrophysiology.
What we learn: Proteome transitions that accompany pruning and synaptogenesis. Biochemical definitions of neuron types and states.
Why it matters: This connects molecules to circuit maturation and behavior at relevant scales. -
Brain aging and cellular resilience
Problem: Decline is preceded by subtle molecular drift. Early detection is actionable.
Approach: Micro-region and single-cell proteomics with redox and energy-pathway readouts.
What we learn: Synaptic and mitochondrial remodeling that precedes behavioral change. Region-specific vulnerabilities and compensations.
Why it matters: This identifies targets for maintaining healthy brain span. -
Addiction biology and plasticity
Problem: Exposure rewires circuits. Lasting molecular changes encode risk and relapse.
Approach: Patch-guided neuron proteomics in reward and stress pathways. Targeted assays for neuropeptides and metabolic mediators.
What we learn: Exposure-dependent proteomes and metabolite signatures that track plasticity.
Why it matters: This exposes biomarkers and mechanisms for prevention and intervention. -
Technology R&D that generalizes
Problem: Biology often fails when the measurement fails.
Approach: Interfaces for low-flow CE–ESI. CE–MS and LC–MS methods for minute inputs. Rapid single-cell analysis. Multiplexed quantification that avoids ratio compression. Real-time logic that conserves sample.
Outcome: Workflows that others can adopt. Results that hold up across instruments and labs.
Current projects (representative)
- Single-cell proteomes during neural induction with functional tests.
- Micro-region proteomics of the circadian pacemaker across maturation.
- Redox-energy mapping in aging circuits with targeted validation.
- Function-linked proteomics of neurons after defined drug exposures.
- Automation and rapid-cycle CE–MS for higher-throughput single-cell studies.
- Cross-platform validation of CE–MS and LC–MS quantification at low input.
REPRESENTATIVE OUTCOMES
A complete list of publications and presentations is available.
Project Example 1: Discovery of fate-altering metabolites in early vertebrate embryos
Goal: Test whether native-level metabolites influence tissue fate before transcript differences appear.
What we did:
- Quantified ~350 metabolites and ~1,700 proteins in mapped single cells of early Xenopus embryos.
- Found dorsal–ventral and left–right asymmetries at the proteome and metabolome levels even when transcripts were similar.
- Identified threonine and histidine enriched in neural-fated cells and acetylcholine and methionine enriched in epidermal-fated cells.
- Microinjected these metabolites at native levels and shifted descendants toward alternate fates in vivo.
Why it matters: Reveals proteo-metabolic control points upstream of gene expression during early patterning.
Figure: Native-level metabolite asymmetries in mapped blastomeres predict and alter tissue fate in vivo.
- Panel A. Cell map of the 16-cell embryo with sampled blastomeres highlighted.
- Panel B. Volcano or bee-swarm plots showing metabolite enrichment by fate group.
- Panel C. Microinjection schematic and representative phenotype images.
Project Example 2: Spemann-Mangold Organizer metabolism and redox signaling during gastrulation
Goal: Define molecular programs that orchestrate the organizer and tissue involution.
What we did:
- Performed lineage-guided sampling of organizer versus non-organizer regions.
- Observed elevated oxidative phosphorylation and localized ROS signatures in organizer tissue.
- Perturbed redox balance and altered morphogenetic movements in functional assays.
Why it matters: Connects energy metabolism and redox control to a century-old problem in embryology.
Figure: Organizer function involves elevated energy metabolism and spatial redox cues that guide morphogenesis.
- Panel A. Schematic of organizer sampling and lineage tracing.
- Panel B. Pathway-level bar plots for OXPHOS and ROS metrics.
- Panel C. Time-lapse stills of tissue involution under redox perturbation.
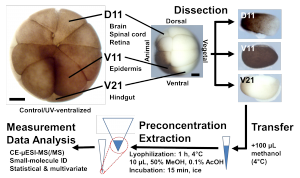
Project Example 3: In vivo single-cell and subcellular proteo-metabolomics
Goal: Measure proteins and metabolites from identified cells in a living embryo while preserving development.
What we did
- Developed in vivo subcellular MS that samples femtoliter-scale volumes without compromising viability.
- Showed that >90% of sampled embryos proceed to normal development and behavior.
- Mapped proteo-metabolic gradients across cells and interfaces that drive polarity and early neural induction.
Why it matters: Enables causal, longitudinal studies of live development with molecular readouts at the right place and time.
Figure: Live, image-guided subcellular sampling yields molecular profiles while preserving normal development.
- Panel A. Microprobe geometry overlaid on a bright-field embryo image.
- Panel B. CE-MS traces from subcellular samples with annotated features.
- Panel C. Developmental outcomes chart comparing sampled versus control embryos.
Project Example 4: Function-linked single-neuron proteomics by Patch-Proteomics
Goal: Relate a neuron’s electrophysiology to its proteome in intact brain tissue.
What we did
- Patched identified neurons in acute mouse brain slices and harvested soma contents.
- Quantified ~200–1,300 proteins from single neurons with CE-MS at trace inputs.
- Classified functionally identified neuronal subtypes by proteome features.
Why it matters: Provides a biochemical definition of neuron types and states directly linked to function.
Figure: Electrophysiology-guided CE-MS reads neuron proteomes and distinguishes functional classes.
- Panel A. Patch-clamp image and sampling workflow.
- Panel B. Protein identifications per neuron and subtype markers.
- Panel C. Dimensionality reduction plot separating functional classes by proteome.
Suggested caption
Project Example 5: Rapid micro-region proteomics in developing brain circuits
Goal: Track proteome transitions in tiny nuclei as circuits mature.
What we did
- Combined CE-ESI-MS with ion mobility and super-resolution microscopy on the suprachiasmatic nucleus.
- From ~10 ng peptide input, identified transitions from axon-guidance proteins pre-eye-opening to synaptic and vesicle-cycling proteins post-eye-opening.
- Validated synapse number increases by imaging without hypertrophy of individual synapses.
Why it matters: Links molecular programs to circuit-level milestones on realistic sample budgets.
Figure: Micro-region proteomics reveals stage-specific protein programs during circuit maturation.
- Panel A. Microdissection schematic and sample mass bar.
- Panel B. Pathway transition heatmap across developmental stages.
- Panel C. Super-resolution images quantifying synapse number changes.
Suggested caption
Project Example 6: Fifteen-minute single-cell proteomics and Eco-AI for accessibility
Goal: Make deep, quantitative single-cell proteomics fast and widely deployable.
What we did
- Implemented 15-minute CE-MS windows with DIA to identify up to ~1,100 proteins from single-cell-equivalent inputs.
- Developed Eco-AI and related electrophoresis-correlative logic to boost identifications and conserve sample on common instruments.
- Demonstrated ~1,800 protein IDs from ~1 ng inputs with real-time guidance and maintained quantitation.
Why it matters: Delivers practical throughput and depth for discovery and screening with scarce material.
Figure: Rapid CE-MS with DIA and Eco-AI increases depth and throughput for small-sample proteomics.
- Panel A. Timeline comparing 15-minute versus conventional runs.
- Panel B. Protein IDs and CVs at single-cell-equivalent inputs.
- Panel C. Schematic of Eco-AI decision logic with example spectra.
Project Example 7: Automation for reproducible nanoscale injections
Goal: Stabilize sample delivery and improve utilization in microanalytical MS.
What we did
- Built RoboCap for pressure-controlled, levitational handling and repeatable injections.
- Improved sample utilization and run-to-run reproducibility compared with manual loading.
- Achieved ~1,800 protein IDs from ~20 ng standards on ion-mobility platforms.
Why it matters: Automation turns expert-only methods into robust workflows others can adopt.
Figure: Robotic handling delivers reproducible nanoscale injections and deeper coverage.
- Panel A. Photo or schematic of RoboCap components.
- Panel B. Injection reproducibility and utilization plots versus manual.
- Panel C. Protein ID boxplots across large run batches.
Project Example 8: Interference-aware deep proteomics in yolk-rich embryos
Goal: Recover informative proteomes when abundant yolk masks signals.
What we did:
- Introduced a yolk-depleted carrier strategy for embryonic tissues.
- Consistently measured >5,000 proteins while reducing MS time spent on yolk peptides.
- Scaled to sparse cell populations using a dilute-to-enrich approach.
Why it matters: Enables deep, quantitative embryonic proteomics where standard workflows fail.
Figure: A yolk-depleted carrier improves depth and quantification in early embryonic proteomics.
- Panel A. Diagram of carrier dilution and sample mixing.
- Panel B. Peptide allocation pie charts with and without the strategy.
- Panel C. Protein ID and quant accuracy summaries across replicates.