By Jerry Brust, IPM Vegetable Specialist, University of Maryland; jbrust@umd.edu
and Karen Rane, Plant Diagnostician, University of Maryland rane@umd.edu
Over the past few weeks we have seen several greenhouse (GH) and high tunnel (HT) vegetable (basil and tomato mostly, but also lettuce, pepper and spinach) operations from around Maryland having problems with thrips. There are several species of vegetable thrips with the most common being the Eastern flower thrips, Frankliniella tritici, Tobacco thrips Frankliniella fusca, Western flower thrips, F. occidentalis and Onion thrips Thrips tabaci.
The last three species are the ones most likely to transmit tomato spotted wilt virus (TSWV). Thrips are tiny, thin yellowish-orange insects the size of metal filings with fringed wings. Thrips have several generations (up to eight) a year. When the weather is warm, the life cycle may be as short as 2 weeks. They feed by puncturing the outer layer of plant tissue and sucking out the cell contents, which results in stippling or discolored flecking that is usually accompanied by black flecks of frass on the damaged areas of the leaf surface.

Thrips feeding damage to basil (A) with flecks of black feces associated with feeding scars (B).

Pepper plant with thrips feeding (A) and TSWV symptoms (B).
Sanitation is one of the most important things that can be done to reduce or eliminate thrips and mite problems from a GH or HT before planting. In most of the cases where we found thrips problems, growers did not follow good sanitation practices. They allowed weeds such as prickly lettuce, chickweed, spiny amaranth, lambsquarters, black nightshade and shepherd’s purse to overwinter in their GH or HT. These weed species not only act as hosts for thrips they also can act as hosts for TSWV. In addition to weeds some growers kept bedding plants in their greenhouse before and at the same time as their vegetable transplants, bedding plants are notorious for harboring thrips. This allows thrips to overwinter and get a head start on the new plantings. Never grow vegetable transplants in the same greenhouse with bedding plants.

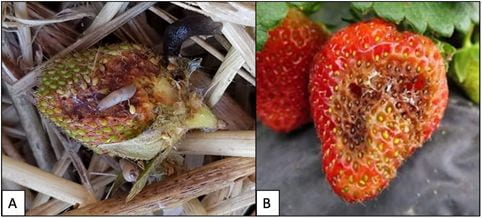
Lettuce leaf with thrips feeding (A) notice how the black flecks follow the feeding scars on the leaf and TSWV symptoms on tomato leaves (B).
Besides the physical damage to leaf and plant tissue several of the thrips can transmit TSWV (Western flower thrips are good at it while Onion thrips and Tobacco thrips are not quite as good at it and Eastern flower thrips cannot transmit the virus). Tomato spotted wilt virus is an obligate parasite, e.g., it must have a living host and must be moved from one plant to another by thrips or through cuttings or possibly seed. This disease can affect tomato and other Solanaceae crops as well as lettuce, beans, cucumber and 170 other plant species. TSWV may occur in the field but tends to affect greenhouse and high tunnel crops more severely. It may take 2 – 4 weeks from when adult thrips first fed on a plant to see initial symptoms occur. Because of this TSWV appears to spread and worsen in plantings over time. TSWV infected leaves may show deformities and mottling or reddish-brown spots or streaks on leaves or stems. Growing tips are usually affected with systemic necrosis and potentially stunted growth.
We tested for both impatiens necrotic spot virus (INSV) and TSWV on tomatoes and bedding plants. Only TSWV was found in both, no INSV was found in any sample. A few growers had some TSWV resistant (or tolerant) tomato varieties (BHN 444 and 640, Dixie Red and Primo Red) and those plants showed no symptoms. However, research shows that the flowers of resistant plants may NOT be resistant, so plants could possibly still become infected with TSWV if thrips feed on the flowers. Several high tunnel vegetable growing operations as well as some GH operations we have seen or that have been reported to us have 20-30% of their plants showing signs of TSWV infection. These plants will not produce much of anything in the way of a harvest and will need to be removed and replaced.
Growers can monitor for thrips using yellow sticky cards that are placed at the same height as the vegetable plants and checking them 2-3 times a week. Early detection can mean using horticultural oils or biological controls for thrips management rather than relying on synthetic chemicals. The biological control agents work best in greenhouse situations and have had mixed results in high tunnel conditions. Predatory mites such as Amblyseius cucumeris or A. swirskii are two good thrips predators. A swirskii works better in warmer temperatures (77-85o F) while A. cucumeris is better in cooler temperatures. A. cucumeris feeds only on first instar larvae so must be released early before thrips populations increase. Orius insidiosus the insidious flower bug, is best used on crops that are producing pollen or by releasing the bugs onto flowering ornamental pepper plants that are in flower which serve as banker plants. Beauveria bassiana, an entomopathogenic fungus that attacks thrips can control thrips problems before they get started by applying weekly applications very early on in the crop cycle in the GH or HT. However, once thrips populations start to rapidly increase a recommended chemical for GH or HT use on the particular vegetable crop should be used. Be sure you know how your state regulates pesticide use in greenhouses and high tunnels. It should be noted that transmission of TSWV may have already taken place by the time even an effective pesticide is used if TSWV infected weeds or plants are present. The 2020-2021 Mid-Atlantic Commercial Vegetable Production Recommendation Guide has recommendations for both GH and HT management of thrips that might vector TSWV.