USDA has just announced that proposed audit fees for the Harmonized and GAP/GHP audits will increase to $155 per hour. The average Harmonized Audit runs anywhere from 12 to 15 hours, GAP/GHP audits average 5 to 10 hours. The current fee is $132 per hour. For anyone who needs an audit try to schedule before October 1, 2023 when the new rates take effect. For further information or discuss the proposed increases contact: Melissa Bailey, Associate Administrator, AMS, USDA, Room 2036–S, 1400 Independence Ave. SW, Washington, DC 20250; telephone (202) 205–9356, or email melissa.bailey@usda.gov.
Month: May 2023
Spinach Crown Mites in Spinach
Spinach Crown Mites in Spinach
by Jerry Brust, UME
Spinach crown mites Rhizoglyphus sp. feed within the folds of new leaves in the crown of spinach plants. This feeding causes the new leaves to become deformed as they grow (figs.1 and 2). Crown mite adults are extremely small bulbous nearly transparent mites that also may have a yellow-beige body color with reddish-brown legs (fig 3). A good characteristic to look for to identify these mites is the sparse long hairs mostly found on the back end of the mite (fig. 3). Crown mite eggs are spherical and clear and laid on the creased leaf surfaces in the crown area. Some reports state that crown mites can act as vectors for plant pathogens such as Pythium and Rhizoctonia, but this is not definitive.

The spinach crown mite is most damaging in soils high in organic matter and under cool moist conditions (weather conditions we have had this past week). Because these mites can consume organic matter they can survive in soils after the crop has been removed. This is one reason they are difficult to control as they can survive for fairly long periods of time with no crop being present. The other reason they are difficult to ‘control’ is we do not realize they are causing the problem until it is too late.

Most control recommendations include sanitation and crop rotations as being important as are fallow periods. Pyrethroids are a possible chemical control as is Neem; any chemical control has to get down into the crown of the plant to have any chance of working. There has been little research conducted on the most efficacious material for these mites. Mostly what is needed are warm sunny days where spinach can grow well and the environment is not so conducive to the mites.
Russet on Apples: Current Understanding and Management
Russet on Apples: Current Understanding and Management
By John Skae, Candidate for B.S. in Physiology and Neurobiology, and Macarena Farcuh, Ph.D. Assistant Professor and Extension Specialist, UMD
What is russet on apples?
Russet on apples is a disorder of the skin that results in discoloration and changes to the expected smooth texture of the skin of apples. Russet appears as a spectrum from mild brown weblike patterns to severe rough changes on the surface of apples and many variations in between (Fig. 1). Russeting is only skin deep and thus will not affect the flesh of the fruit. It can occur due to naturally-occurring weather conditions, particularly humid and wet weather.
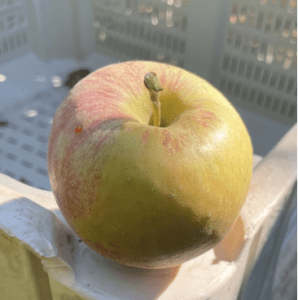
Russeting can ultimately reduce the market value of apples, decreasing grower profitability. According to the US Department of Agriculture, the presence of russet disqualifies apples from the US Extra Fancy, US Fancy grades if smooth, solid russeting is more than 10%, while excessive russeting or smooth net-like russeting exceeding 25% excludes fruit from the US No. 1 grade categories.
Apples can begin to russet within the first 30-40 days of development, starting at petal fall. The earlier the tissues are damaged, the more dramatic the damage will be. But it is important to mention that russet can also be developed later during the growing season. Russeting occurs because cracks begin to develop underneath the cuticle of the apple. The damage in the epidermal cells underneath the cuticle turns brown. The cells are then pushed upwards towards the skin because new cork cells are growing underneath the affected epidermal cells. Once the damaged cells reach the surface of the apple, they form phellogen, a wound-sealing tissue created as a result of the russeting reaching the surface. Although russeting affects the cosmetic appearance of apples, it does not harm fruit flesh taste.
What factors cause or contribute to apple russeting?
Some cultivars produced by selective breeding are more prone to russeting than others as these can alter the genetic makeup of the apple to express russet. For example, Golden Delicious apples are highly susceptible to russet, while Red Delicious apples rarely express the russeting disorder.
Continue reading Russet on Apples: Current Understanding and Management
White Rot of Onion and Garlic
White Rot of Onion and Garlic
By Jerry Brust, UME and Karen Rane, UMD Plant Diagnostic Lab
One very serious soil disease that affects Allium species, especially onion and garlic, is white rot, caused by the fungus Stromatinia cepivorum (syn. Sclerotium cepivorum (fig. 1)). White rot is NOT the same as white mold, which is caused by Sclerotinia sclerotiorum, which has a very large host range (tomatoes, peppers and 170 other plant species); white rot only infects Allium species.

Leaves of Allium plants with white rot exhibit yellowing, dieback, and wilting. Under ideal weather conditions, white mycelial growth can develop on the bulb. As the disease progresses, the mycelium becomes more compacted with numerous small, spherical black bodies (sclerotia) forming on this white mat (fig. 2). These sclerotia are the overwintering structures of the pathogen and are approximately the size of a pin head. As the disease progresses, these sclerotia are eventually released into the soil. Infected plant roots will rot, making the plant easily pulled from the soil. Disease development is favored by cool, moist soil conditions. The soil temperature range for infection is 50°-75°F, with an optimum of 60°- 65°F. At soil temperatures above 78°F, the disease is greatly inhibited. Soil moisture conditions that are favorable for onion and garlic growth are also best for white rot development.

An increase of white rot in a field that has had several Allium crops may go unnoticed for a time as sclerotia numbers increase and disperse. One sclerotium per 20 pounds of soil will cause disease and results in measurable crop loss. The sclerotia will lay dormant until root exudates, exclusively from an Allium species, stimulate germination. Root exudates from non-Allium species will not stimulate the germination of white rot sclerotia. Cool weather is needed for both sclerotia germination and mycelia growth. Mycelia will grow through the soil until they encounter an Allium root at which time the fungus initiates infection. Mycelia can grow from one plant to a nearby plant, allowing the pathogen to move between plants.
Management of white rot should focus on disease avoidance by not introducing the pathogen into a field. Sclerotia can spread throughout a field, or from field to field, through the movement of soil, equipment, or plant material (especially garlic cloves). Sanitation is important to prevent sclerotia from moving from an infested field to a clean field. Plant only clean stock from known origins that has no history of white rot. Always clean soil off of equipment and sanitize with quaternary ammonia before moving to another field. The Allium crops from an infested field should not be used as seed. Rotation alone will not control white rot because sclerotia can survive in the soil for 20-40 years. If the disease is found, reducing or eliminating irrigation will reduce the damage to the current crop but will not stop the spread of the disease.
Because the fungus is vulnerable to temperatures above 115°F, dipping seed garlic in hot water is a possible preventive measure that will reduce the amount of pathogen but will not completely eliminate it. Temperature control is important when using this method because temperatures above 120°F may kill the garlic. There are other cultural and organic practices (i.e., biofumigation and solarization) that a grower might try to fight this disease and these can be found at: https://rvpadmin.cce.cornell.edu/uploads/doc_479.pdf
Chemical applications can be made for white rot management and include for onion tebuconazole applied in a 4-6 inch band over or into the furrow at planting or via chemigation. For garlic an in-furrow at-planting application using iprodione or tebuconazole or fludioxonil can reduce disease incidence, however there are crop rotation restrictions with the use of these chemicals so be sure to check the Mid-Atlantic Commercial Production Recommendations guide for more details.
One other note is that the presence of bulb mites can exacerbate disease problems by opening the bulb up to infection from white rot and growers also will need to manage these mites.
Check for Allium Leaf Miner in Onions and Leeks
Check for Allium Leaf Miner in Onions and Leeks
By Jerry Brust, IPM Vegetable Specialist UME
If you grow leeks or onions or other Allium species, you should already be checking for the tell-tale marks left by Allium leaf miner. Allium leaf miner Phytomyza gymnostoma tell-tale marks consist of many linear small white dots (made by the female’s ovipositor) that appear in the middle towards the end of leaf blades (fig. 1) of their preferred hosts of leeks, onions, garlic and other Allium species. Spring crops are usually not as hard hit as fall crops especially when looking at leeks, but this pest has been steadily increasing its geographical range each year as well as its damage potential. If you had some infestation last year you will especially want to be looking for the signs of this pest.

To go over recommendations for this pest: New transplants or seedings of onions, leeks or garlic should be watched closely for the tell-tale signs of the fly’s damage. When eggs hatch the larvae at first mine leaves (fig. 2) and then move down to the bulbs and leaf sheathes where they feed and eventually pupate. Pupae will undergo a summer aestivation (type of hibernation because temperatures are too warm for them to be active) and only emerge again in late September. You can cover any just-transplanted Allium planting with a row cover (but don’t wait too long after transplanting) to keep the flies off or if needed treat with insecticides. Research out of Cornell University has found using just two applications of spinosad (Entrust, which is OMRI-labelled) two weeks after oviposition marks are first found and then another application 2 weeks after this will give adequate control of the pest. But the oviposition marks must be watched for carefully and discovered very soon after first being made. If new oviposition marks are being seen each week a weekly application of insecticide may be necessary. A penetrant adjuvant also is recommended to be used when treating for the larvae.
